Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: an exploratory randomized phase II trial
- PMID: 31386014
- PMCID: PMC6655481
- DOI: 10.1093/nop/npw025
Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: an exploratory randomized phase II trial
Abstract
Background: In patients with recurrent glioblastoma, the benefit of bevacizumab beyond progression remains uncertain. We prospectively evaluated continuing or ceasing bevacizumab in patients who progressed while on bevacizumab.
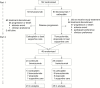
Methods: CABARET, a phase II study, initially randomized patients to bevacizumab with or without carboplatin (Part 1). At progression, eligible patients underwent a second randomization to continue or cease bevacizumab (Part 2). They could also receive additional chemotherapy regimens (carboplatin, temozolomide, or etoposide) or supportive care.
Results: Of 120 patients treated in Part 1, 48 (80% of the anticipated 60-patient sample size) continued to Part 2. Despite randomization, there were some imbalances in patient characteristics. The best response was stable disease in 7 (30%) patients who continued bevacizumab and 2 (8%) patients who stopped receiving bevacizumab. There were no radiological responses. Median progression-free survival was 1.8 vs 2.0 months (bevacizumab vs no bevacizumab; hazard ratio [HR], 1.08; 95% CI, .59-1.96; P = .81). Median overall survival was 3.4 vs 3.0 months (HR, .84; 95% CI, .47-1.50; P = .56 and HR .70; 95% CI .38-1.29; P = .25 after adjustment for baseline factors). Quality-of-life scores did not significantly differ between arms. While the maximum daily steroid dose was lower in the continuation arm, the difference was not statistically significant.
Conclusions: Patients who continued bevacizumab beyond disease progression did not have clear survival improvements, although the study was not powered to detect other than very large differences. While these data provide the only randomized evidence related to continuing bevacizumab beyond progression in recurrent glioblastoma, the small sample size precludes definitive conclusions and suggests this remains an open question.
Keywords: bevacizumab; carboplatin; glioblastoma; phase II trial.
Figures
References
-
- Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106(3):309–312. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503; quiz 1 p following 516. - PubMed
-
- Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. - PubMed