Ghrelin ameliorates the phenotype of newborn rats induced with mild necrotizing enterocolitis
- PMID: 31386261
- PMCID: PMC6791725
- DOI: 10.1111/nmo.13682
Ghrelin ameliorates the phenotype of newborn rats induced with mild necrotizing enterocolitis
Abstract
Background: We have shown previously that an attenuated rodent model of mild necrotizing enterocolitis (NEC) increases intestinal histopathological severity grade, prevents typical developmental increases in the high-frequency spectrum of heart rate variability (HF-HRV), alters the nitrergic myenteric phenotype, and increases IL-6 and IL-1β when combined with anterior subdiaphragmatic vagotomy. The aims of the present study were to test the hypotheses that in mild NEC-induced pups, administration of the orexigenic hormone ghrelin (a) reduces the histopathological score, (b) increases the HF-HRV power, (c) improves the altered myenteric phenotype, and (d) subdiaphragmatic vagotomy prevents the effects of ghrelin.
Methods: Newborn Sprague Dawley rats were subjected to seven days of brief periods of cold stress and hypoxia to induce mild NEC with or without anterior subdiaphragmatic vagotomy. HRV was measured at postnatal days one, five, and ten; intraperitoneal ghrelin (0.05 mg kg-1 ) was administered postnatal days five through ten b.i.d. Pups were sacrificed at day 12, and whole brains, gastrointestinal tissues, and blood were collected for immunohistochemical, corticosterone, and cytokine analysis.
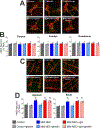
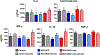
Key results: Ghrelin treatment reduced the intestinal histopathological score, increased the HF-HRV power, improved the altered intestinal myenteric phenotype, and subdiaphragmatic vagotomy prevented the effects of ghrelin. There were no differences in serum cytokines or corticosterone between groups.
Conclusions and inferences: Our data suggest that ghrelin administration is able to recover the mild NEC-induced changes to the histology, HF-HRV, and myenteric phenotype in a vagally dependent manner.
Keywords: enteric nervous system; heart rate variability; immunohistochemistry; vagus.
© 2019 John Wiley & Sons Ltd.
Figures


References
-
- Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep. 2008;10(5):450–457. - PubMed
-
- Luig M, Lui K, Nsw, Group AN. Epidemiology of necrotizing enterocolitis--Part II: Risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41(4):174–179. - PubMed
-
- Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

