Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children's Oncology Group Study ANBL0531
- PMID: 31386611
- PMCID: PMC6881103
- DOI: 10.1200/JCO.19.00919
Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children's Oncology Group Study ANBL0531
Abstract
Purpose: The primary objective of the Children's Oncology Group study ANBL0531 (ClinicalTrials.gov identifier: NCT00499616) was to reduce therapy for subsets of patients with intermediate-risk neuroblastoma using a biology- and response-based algorithm to assign treatment duration while maintaining a 3-year overall survival (OS) of 95% or more for the entire cohort.
Patients and methods: Children younger than age 12 years with intermediate-risk stage 2A/2B or stage 3 tumors with favorable histology; infants younger than age 365 days with stage 3, 4 or 4S disease; and toddlers from 365 to younger than 547 days with favorable histology, hyperdiploid stage 4, or unfavorable histology stage 3 tumors were eligible. Patients with MYCN-amplified tumors were excluded. Patients were assigned to initially receive two (group 2), four (group 3), or eight (group 4) cycles of chemotherapy with or without surgery on the basis of prognostic markers, including allelic status of chromosomes 1p and 11q; ultimate duration of therapy was determined by overall response.
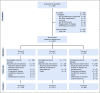
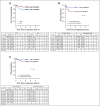
Results: Between 2007 and 2011, 404 evaluable patients were enrolled. Compared with legacy Children's Oncology Group studies, subsets of patients had a reduction in treatment. The 3-year event-free survival and OS rates were 83.2% (95% CI, 79.4% to 87.0%) and 94.9% (95% CI, 92.7% to 97.2%), respectively. Infants with stage 4 tumors with favorable biology (n = 61) had superior 3-year event-free survival compared with patients with one or more unfavorable biologic features (n = 47; 86.9% [95% CI, 78.3% to 95.4%] v 66.8% [95% CI, 53.1% to 80.6%]; P = .02), with a trend toward OS advantage (95.0% [95% CI, 89.5% to 100%] v 86.7% [95% CI, 76.6% to 96.7%], respectively; P = .08). OS for patients with localized disease was 100%.
Conclusion: Excellent survival was achieved with this treatment algorithm, with reduction of therapy for subsets of patients. More-effective treatment strategies still are needed for infants with unfavorable biology stage 4 disease.
Figures

References
-
- Irwin MS, Park JR. Neuroblastoma: Paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. - PubMed

