Strategic deployment of feature-based attentional gain in primate visual cortex
- PMID: 31386656
- PMCID: PMC6684042
- DOI: 10.1371/journal.pbio.3000387
Strategic deployment of feature-based attentional gain in primate visual cortex
Abstract
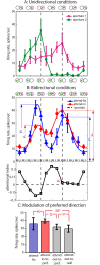
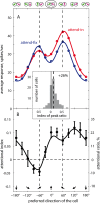
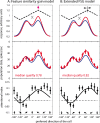
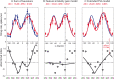
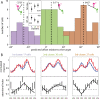
Attending to visual stimuli enhances the gain of those neurons in primate visual cortex that preferentially respond to the matching locations and features (on-target gain). Although this is well suited to enhance the neuronal representation of attended stimuli, it is nonoptimal under difficult discrimination conditions, as in the presence of similar distractors. In such cases, directing attention to neighboring neuronal populations (off-target gain) has been shown to be the most efficient strategy, but although such a strategic deployment of attention has been shown behaviorally, its underlying neural mechanisms are unknown. Here, we investigated how attention affects the population responses of neurons in the middle temporal (MT) visual area of rhesus monkeys to bidirectional movement inside the neurons' receptive field (RF). The monkeys were trained to focus their attention onto the fixation spot or to detect a direction or speed change in one of the motion directions (the "target"), ignoring the distractor motion. Population activity profiles were determined by systematically varying the patterns' directions while maintaining a constant angle between them. As expected, the response profiles show a peak for each of the 2 motion directions. Switching spatial attention from the fixation spot into the RF enhanced the peak representing the attended stimulus and suppressed the distractor representation. Importantly, the population data show a direction-dependent attentional modulation that does not peak at the target feature but rather along the slopes of the activity profile representing the target direction. Our results show that attentional gains are strategically deployed to optimize the discriminability of target stimuli, in line with an optimal gain mechanism proposed by Navalpakkam and Itti.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Feature-based attention increases the selectivity of population responses in primate visual cortex.Curr Biol. 2004 May 4;14(9):744-51. doi: 10.1016/j.cub.2004.04.028. Curr Biol. 2004. PMID: 15120065
-
Frequency-dependent attentional modulation of local field potential signals in macaque area MT.J Neurosci. 2010 May 19;30(20):7037-48. doi: 10.1523/JNEUROSCI.0404-10.2010. J Neurosci. 2010. PMID: 20484646 Free PMC article.
-
Effects of attention and distractor contrast on the responses of middle temporal area neurons to transient motion direction changes.Eur J Neurosci. 2015 Jun;41(12):1603-13. doi: 10.1111/ejn.12920. Epub 2015 May 15. Eur J Neurosci. 2015. PMID: 25885809
-
Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas.J Neurosci. 1999 Sep 1;19(17):7591-602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. J Neurosci. 1999. PMID: 10460265 Free PMC article.
-
A review of the mechanisms by which attentional feedback shapes visual selectivity.Brain Struct Funct. 2015;220(3):1237-50. doi: 10.1007/s00429-014-0818-5. Epub 2014 Jul 3. Brain Struct Funct. 2015. PMID: 24990408 Free PMC article. Review.
Cited by
-
Coupled oscillations orchestrate selective information transmission in visual cortex.PNAS Nexus. 2024 Jul 31;3(8):pgae288. doi: 10.1093/pnasnexus/pgae288. eCollection 2024 Aug. PNAS Nexus. 2024. PMID: 39161729 Free PMC article.
-
Attention to visual motion suppresses neuronal and behavioral sensitivity in nearby feature space.BMC Biol. 2022 Oct 5;20(1):220. doi: 10.1186/s12915-022-01428-7. BMC Biol. 2022. PMID: 36199136 Free PMC article.
-
Attentional priority is determined by predicted feature distributions.J Exp Psychol Hum Percept Perform. 2022 Nov;48(11):1201-1212. doi: 10.1037/xhp0001041. Epub 2022 Sep 1. J Exp Psychol Hum Percept Perform. 2022. PMID: 36048065 Free PMC article.
-
Commentary: The causal role of α-oscillations in feature binding.Front Neurosci. 2020 Aug 19;14:753. doi: 10.3389/fnins.2020.00753. eCollection 2020. Front Neurosci. 2020. PMID: 32973423 Free PMC article. No abstract available.
-
Histological assessment of optogenetic tools to study fronto-visual and fronto-parietal cortical networks in the rhesus macaque.Sci Rep. 2020 Jul 6;10(1):11051. doi: 10.1038/s41598-020-67752-6. Sci Rep. 2020. PMID: 32632196 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

