Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis
- PMID: 31386659
- PMCID: PMC6684046
- DOI: 10.1371/journal.pmed.1002848
Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis
Abstract
Background: Metformin is increasingly offered as an acceptable and economic alternative to insulin for treatment of gestational diabetes mellitus (GDM) in many countries. However, the impact of maternal metformin treatment on the trajectory of fetal, infant, and childhood growth is unknown.
Methods and findings: PubMed, Ovid Embase, Medline, Web of Science, ClinicalTrials.gov, and the Cochrane database were systematically searched (from database inception to 26 February 2019). Outcomes of GDM-affected pregnancies randomised to treatment with metformin versus insulin were included (randomised controlled trials and prospective randomised controlled studies) from cohorts including European, American, Asian, Australian, and African women. Studies including pregnant women with pre-existing diabetes or non-diabetic women were excluded, as were trials comparing metformin treatment with oral glucose-lowering agents other than insulin. Two reviewers independently assessed articles for eligibility and risk of bias, and conflicts were resolved by a third reviewer. Outcome measures were parameters of fetal, infant, and childhood growth, including weight, height, BMI, and body composition. In total, 28 studies (n = 3,976 participants) met eligibility criteria and were included in the meta-analysis. No studies reported fetal growth parameters; 19 studies (n = 3,723 neonates) reported measures of neonatal growth. Neonates born to metformin-treated mothers had lower birth weights (mean difference -107.7 g, 95% CI -182.3 to -32.7, I2 = 83%, p = 0.005) and lower ponderal indices (mean difference -0.13 kg/m3, 95% CI -0.26 to 0.00, I2 = 0%, p = 0.04) than neonates of insulin-treated mothers. The odds of macrosomia (odds ratio [OR] 0.59, 95% CI 0.46 to 0.77, p < 0.001) and large for gestational age (OR 0.78, 95% CI 0.62 to 0.99, p = 0.04) were lower following maternal treatment with metformin compared to insulin. There was no difference in neonatal height or incidence of small for gestational age between groups. Two studies (n = 411 infants) reported measures of infant growth (18-24 months of age). In contrast to the neonatal phase, metformin-exposed infants were significantly heavier than those in the insulin-exposed group (mean difference 440 g, 95% CI 50 to 830, I2 = 4%, p = 0.03). Three studies (n = 520 children) reported mid-childhood growth parameters (5-9 years). In mid-childhood, BMI was significantly higher (mean difference 0.78 kg/m2, 95% CI 0.23 to 1.33, I2 = 7%, p = 0.005) following metformin exposure than following insulin exposure, although the difference in absolute weights between the groups was not significantly different (p = 0.09). Limited evidence (1 study with data treated as 2 cohorts) suggested that adiposity indices (abdominal [p = 0.02] and visceral [p = 0.03] fat volumes) may be higher in children born to metformin-treated compared to insulin-treated mothers. Study limitations include heterogeneity in metformin dosing, heterogeneity in diagnostic criteria for GDM, and the scarcity of reporting of childhood outcomes.
Conclusions: Following intrauterine exposure to metformin for treatment of maternal GDM, neonates are significantly smaller than neonates whose mothers were treated with insulin during pregnancy. Despite lower average birth weight, metformin-exposed children appear to experience accelerated postnatal growth, resulting in heavier infants and higher BMI by mid-childhood compared to children whose mothers were treated with insulin. Such patterns of low birth weight and postnatal catch-up growth have been reported to be associated with adverse long-term cardio-metabolic outcomes. This suggests a need for further studies examining longitudinal perinatal and childhood outcomes following intrauterine metformin exposure. This review protocol was registered with PROSPERO under registration number CRD42018117503.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




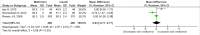
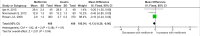
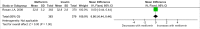
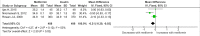
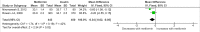
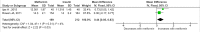
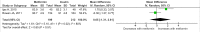
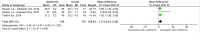


References
-
- Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66:14–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

