Home rehabilitation supported by a wearable soft-robotic device for improving hand function in older adults: A pilot randomized controlled trial
- PMID: 31386685
- PMCID: PMC6684161
- DOI: 10.1371/journal.pone.0220544
Home rehabilitation supported by a wearable soft-robotic device for improving hand function in older adults: A pilot randomized controlled trial
Abstract
Background: New developments, based on the concept of wearable soft-robotic devices, make it possible to support impaired hand function during the performance of daily activities and intensive task-specific training. The wearable soft-robotic ironHand glove is such a system that supports grip strength during the performance of daily activities and hand training exercises at home.
Design: This pilot randomized controlled clinical study explored the effect of prolonged use of the assistive ironHand glove during daily activities at home, in comparison to its use as a trainings tool at home, on functional performance of the hand.
Methods: In total, 91 older adults with self-perceived decline of hand function participated in this study. They were randomly assigned to a 4-weeks intervention of either assistive or therapeutic ironHand use, or control group (received no additional exercise or treatment). All participants performed a maximal pinch grip test, Box and Blocks test (BBT), Jebsen-Taylor Hand Function Test (JTHFT) at baseline and after 4-weeks of intervention. Only participants of the assistive and therapeutic group completed the System Usability Scale (SUS) after the intervention period.
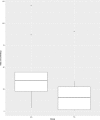
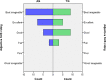
Results: Participants of the assistive and therapeutic group reported high scores on the SUS (mean = 73, SEM = 2). The therapeutic group showed improvements in unsupported handgrip strength (mean Δ = 3) and pinch strength (mean Δ = 0.5) after 4 weeks of ironHand use (p≤0.039). Scores on the BBT and JTHFT improved not only after 4 weeks of ironHand use (assistive and therapeutic), but also in the control group. Only handgrip strength improved more in the therapeutic group compared to the assistive and control group. No significant correlations were found between changes in performance and assistive or therapeutic ironHand use (p≥0.062).
Conclusion: This study showed that support of the wearable soft-robotic ironHand system either as assistive device or as training tool may be a promising way to counter functional hand function decline associated with ageing.
Conflict of interest statement
The authors have declared that no competing interests exist. Potential conflict of interests: Our project partners Bioservo Technologies AB and Hocoma AG were involved in the ironHand project (grant AAL-2013-6-134), and interested in commercializing the ironHand system. Alejandro Melendez- Calderon was with Hocoma AG at the time of the study, but is now with Cereneo AG, Switzerland. He does not receive any financial or personal benefits from Hocoma AG or any other source for the publication of this study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Carmeli E, Patish H, Coleman R. The aging hand. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(2):M146–M52. - PubMed
-
- Hunter S, Crome P. Hand function and stroke. Reviews in Clinical Gerontology. 2002;12(01):68–81.
-
- Desrosiers J, Hébert R, Bravo G, Rochette A. Age-related changes in upper extremity performance of elderly people: a longitudinal study. Experimental gerontology. 1999;34(3):393–405. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

