Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: A novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function
- PMID: 31386965
- PMCID: PMC6692063
- DOI: 10.1016/j.redox.2019.101287
Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: A novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function
Abstract
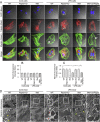
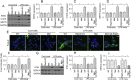
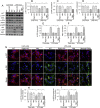
The basic pathophysiological mechanisms underlying septic cardiomyopathy have not yet been completely clarified. Disease-specific treatments are lacking, and care is still based on supportive modalities. The aim of our study was to assess the protective effects of melatonin on septic cardiomyopathy, with a focus on the interactions between receptor-interacting protein kinase 3 (Ripk3), the mitochondria, endoplasmic reticulum (ER) and cytoskeletal degradation in cardiomyocytes. Ripk3 expression was increased in heart samples challenged with LPS, followed by myocardial inflammation, cardiac dysfunction, myocardial breakdown and cardiomyocyte death. The melatonin treatment attenuated septic myocardial injury in a comparable manner to the genetic depletion of Ripk3. Molecular investigations revealed that Ripk3 intimately regulated mitochondrial function, ER stress, cytoskeletal homeostasis and cardioprotective signaling pathways. Melatonin-mediated inhibition of Ripk3 improved mitochondrial bioenergetics, reduced mitochondria-initiated oxidative damage, sustained mitochondrial dynamics, ameliorated ER stress, normalized calcium recycling, and activated cardioprotective signaling pathways (including AKT, ERK and AMPK) in cardiomyocytes. Interestingly, Ripk3 overexpression mediated resistance to melatonin therapy following the infection of LPS-treated hearts with an adenovirus expressing Ripk3. Altogether, our findings identify Ripk3 upregulation as a novel risk factor for the development of sepsis-related myocardial injury, and melatonin restores the physiological functions of the mitochondria, ER, contractile cytoskeleton and cardioprotective signaling pathways. Additionally, our data also reveal a new, potentially therapeutic mechanism by which melatonin protects the heart from sepsis-mediated dysfunction, possibly by targeting Ripk3.
Keywords: Cardioprotective signaling pathways; ER stress; Melatonin; Mitochondrial injury; Ripk3; Septic cardiomyopathy.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Aanhane E., Schulkens I.A., Heusschen R., Castricum K., Leffler H., Griffioen A.W., Thijssen V.L. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis. 2018;21(3):545–555. - PubMed
-
- Abukar Y., Ramchandra R., Hood S.G., Mckinley M.J., Booth L.C., Yao S.T., May C.N. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res. Cardiol. 2018;113(5):35. - PubMed
-
- Abeysuriya R.G., Lockley S.W., Robinson P.A., Postnova S. A unified model of melatonin, 6-sulfatoxymelatonin, and sleep dynamics. J. Pineal Res. 2018;64(4) - PubMed
-
- Frencken J.F., Donker D.W., Spitoni C., Koster-Brouwer M.E., Soliman I.W., Ong D.S.Y., Horn J., Van Der Poll T., Van Klei W.A., Bonten M.J.M., Cremer O.L. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11(2) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

