Iron Regulation by Molidustat, a Daily Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor, in Patients with Chronic Kidney Disease
- PMID: 31387097
- PMCID: PMC6979436
- DOI: 10.1159/000502012
Iron Regulation by Molidustat, a Daily Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor, in Patients with Chronic Kidney Disease
Abstract
Background/aims: The current treatment for anemia associated with chronic kidney disease (CKD) includes the administration of erythropoiesis stimulating agents (ESAs) combined with iron supplementation. Molidustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor, has potential to treat anemia associated with CKD through increased erythropoietin production and improved iron availability. Here, we report the effect of molidustat on iron metabolism.
Method: Parameters of iron metabolism were monitored in three 16-week, randomized, controlled, phase 2 studies assessing the safety and efficacy of molidustat in the treatment of anemia associated with CKD in different populations: treatment-naïve and previously ESA-treated patients not on dialysis, and previously ESA-treated patients on hemodialysis. Iron supplementation was left at the discretion of the investigator.
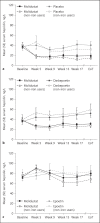
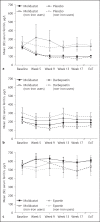
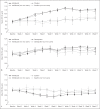
Results: In treatment-naïve patients not on dialysis, transferrin saturation (TSAT), hepcidin, ferritin, and iron concentrations decreased with molidustat, whereas total iron binding capacity (TIBC) increased. Similar results were observed in previously ESA-treated patients not on dialysis, although changes in those parameters were larger in treatment-naïve than in previously ESA-treated patients. In previously ESA-treated patients receiving hemodialysis, hepcidin concentration and TIBC remained stable with molidustat, whereas TSAT and ferritin and iron concentrations increased. Generally, similar trends were observed in secondary analyses of subgroups of patients not receiving iron supplementation.
Conclusions: Molidustat is a potential alternative to standard treatment of anemia associated with CKD, with a different mechanism of action. In patients not receiving dialysis, molidustat increases iron availability. In patients receiving hemodialysis, further investigation is required to understand fully the mechanisms underlying iron mobilization associated with molidustat.
Keywords: Anemia; Chronic kidney disease; Hypoxia-inducible factor prolyl hydroxylase inhibitor; Iron metabolism; Molidustat.
© 2019 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
T.A.: has received consulting fees from Astellas, Bayer Yakuhin Ltd., GlaxoSmithKline, J.T. Pharmaceuticals, Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd., and Ono Pharmaceutical Co. Ltd., and lecture fees from Bayer Yakuhin Ltd., Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, and Torii Pharmaceutical Co. Ltd. I.C.M. has received research funding for the DIALOGUE studies, honoraria for steering committee activities, and speaker fees from Bayer Pharma AG; and has received research support and speakers' honoraria from Akebia, Astellas, FibroGen, and GlaxoSmithKline. J.S.B. has served on the executive committees for the DIALOGUE studies and for an Amgen-sponsored darbepoetin clinical trial. M.T. and K.I. are employees of Bayer Yakuhin Ltd. T.B. is an employee of Bayer AG.
Figures
References
-
- van Nooten FE, Green J, Brown R, Finkelstein FO, Wish J. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States review of the literature. J Med Econ. 2010;13((2)):241–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

