Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue
- PMID: 31387210
- PMCID: PMC6721723
- DOI: 10.3390/cells8080830
Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue
Abstract
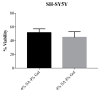
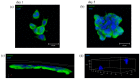
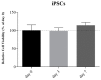
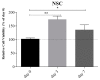
Neurodegenerative diseases (NDs) are a broad class of pathologies characterized by the progressive loss of neurons in the central nervous system. The main problem in the study of NDs is the lack of an adequate realistic experimental model to study the pathogenic mechanisms. Induced pluripotent stem cells (iPSCs) partially overcome the problem, with their capability to differentiate into almost every cell types; even so, these cells alone are not sufficient to unveil the mechanisms underlying NDs. 3D bioprinting allows to control the distribution of cells such as neurons, leading to the creation of a realistic in vitro model. In this work, we analyzed two biomaterials: sodium alginate and gelatin, and three different cell types: a neuroblastoma cell line (SH-SY5Y), iPSCs, and neural stem cells. All cells were encapsulated inside the bioink, printed and cultivated for at least seven days; they all presented good viability. We also evaluated the maintenance of the printed shape, opening the possibility to obtain a reliable in vitro neural tissue combining 3D bioprinting and iPSCs technology, optimizing the study of the degenerative processes that are still widely unknown.
Keywords: 3D bioprinting; 3D cell culture; bioink; cell culture; disease modeling; gelatin; iPSC; neural stem cell; neuroblastoma cell line; sodium alginate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells.Biofabrication. 2016 Sep 16;8(3):035020. doi: 10.1088/1758-5090/8/3/035020. Biofabrication. 2016. PMID: 27634915
-
3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation.Adv Healthc Mater. 2017 Sep;6(17). doi: 10.1002/adhm.201700175. Epub 2017 May 24. Adv Healthc Mater. 2017. PMID: 28544655
-
Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells.Adv Healthc Mater. 2016 Jun;5(12):1429-38. doi: 10.1002/adhm.201600095. Epub 2016 Mar 29. Adv Healthc Mater. 2016. PMID: 27028356
-
ECM Based Bioink for Tissue Mimetic 3D Bioprinting.Adv Exp Med Biol. 2018;1064:335-353. doi: 10.1007/978-981-13-0445-3_20. Adv Exp Med Biol. 2018. PMID: 30471042 Review.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
3D Bioprinting of Neural Tissues.Adv Healthc Mater. 2021 Aug;10(15):e2001600. doi: 10.1002/adhm.202001600. Epub 2020 Nov 16. Adv Healthc Mater. 2021. PMID: 33200587 Free PMC article. Review.
-
The Evolution of Technology-Driven In Vitro Models for Neurodegenerative Diseases.Adv Sci (Weinh). 2024 Apr;11(16):e2304989. doi: 10.1002/advs.202304989. Epub 2024 Feb 17. Adv Sci (Weinh). 2024. PMID: 38366798 Free PMC article. Review.
-
Design and biofabrication of bacterial living materials with robust and multiplexed biosensing capabilities.Mater Today Bio. 2022 Dec 24;18:100526. doi: 10.1016/j.mtbio.2022.100526. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36632629 Free PMC article.
-
3D Bioprinting of Neurovascular Tissue Modeling with Collagen-Based Low-Viscosity Composites.Adv Healthc Mater. 2023 Oct;12(25):e2300004. doi: 10.1002/adhm.202300004. Epub 2023 Jun 14. Adv Healthc Mater. 2023. PMID: 37264745 Free PMC article.
-
Perineural Invasion in Adenoid Cystic Carcinoma of the Salivary Glands: Where We Are and Where We Need to Go.Front Oncol. 2020 Aug 18;10:1493. doi: 10.3389/fonc.2020.01493. eCollection 2020. Front Oncol. 2020. PMID: 33014792 Free PMC article. Review.
References
-
- Csobonyeiova M., Polak S., Nicodemou A., Danisovic L. Induced pluripotent stem cells in modeling and cell-based therapy of amyotrophic lateral sclerosis. J. Physiol. Pharm. 2017;68:649–657. - PubMed

