Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity
- PMID: 31387329
- PMCID: PMC6723184
- DOI: 10.3390/biom9080346
Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity
Abstract
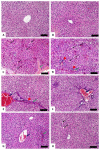
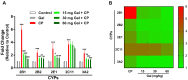
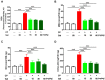
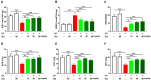
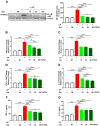
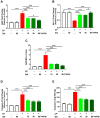
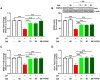
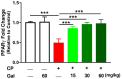
Cyclophosphamide (CP) is a widely used chemotherapeutic agent; however, its clinical application is limited because of its multi-organ toxicity. Galangin (Gal) is a bioactive flavonoid with promising biological activities. This study investigated the hepatoprotective effect of Gal in CP-induced rats. Rats received Gal (15, 30 and 60 mg/kg/day) for 15 days followed by a single dose of CP at day 16. Cyclophosphamide triggered liver injury characterized by elevated serum transaminases, alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), and histopathological manifestations. Increased hepatic reactive oxygen species, malondialdehyde, nitric oxide, and oxidative DNA damage along with declined glutathione and antioxidant enzymes were demonstrated in CP-administered rats. CP provoked hepatic nuclear factor-kappaB (NF-κB) phosphorylation and increased mRNA abundance of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) both expression and serum levels. Gal prevented CP-induced liver injury, boosted antioxidants and suppressed oxidative stress, DNA damage, NF-κB phosphorylation and pro-inflammatory mediators. Gal diminished Bax and caspase-3, and increased B-cell lymphoma-2 (Bcl-2) in liver of CP-administered rats. In addition, Gal increased peroxisome proliferator-activated receptor gamma (PPARγ) expression and activated hepatic nuclear factor erythroid 2-related factor 2 (Nrf2) signaling showed by the increase in Nrf2, NAD(P)H: quinone acceptor oxidoreductase-1 (NQO-1) and heme oxygenase 1 (HO-1) in CP-administered rats. These findings suggest that Gal prevents CP hepatotoxicity through activation of Nrf2/HO-1 signaling and attenuation of oxidative damage, inflammation and cell death. Therefore, Gal might represent a promising adjuvant therapy to prevent hepatotoxicity in patients on CP treatment.
Keywords: cyclophosphamide; galangin; hepatotoxicity; inflammation; nuclear factor erythroid 2-related factor 2; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

