A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues
- PMID: 31387330
- PMCID: PMC6695859
- DOI: 10.3390/ijms20153832
A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues
Abstract
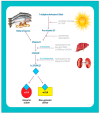
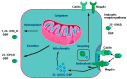
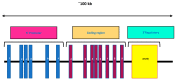
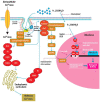
Vitamin D is a major steroid hormone that is gaining attention as a therapeutic molecule. Due to the general awareness of its importance for the overall well-being, vitamin D deficiency (VDD) is now recognized as a major health issue. The main reason for VDD is minimal exposure to sunlight. The vitamin D receptor (VDR) is a member of the steroid hormone receptors that induces a cascade of cell signaling to maintain healthy Ca2+ levels that serve to regulate several biological functions. However, the roles of vitamin D and its metabolism in maintaining gastric homeostasis have not yet been completely elucidated. Currently, there is a need to increase the vitamin D status in individuals worldwide as it has been shown to improve musculoskeletal health and reduce the risk of chronic illnesses, including some cancers, autoimmune and infectious diseases, type 2 diabetes mellitus, neurocognitive disorders, and general mortality. The role of vitamin D in gastric homeostasis is crucial and unexplored. This review attempts to elucidate the central role of vitamin D in preserving and maintaining the overall health and homeostasis of the stomach tissue.
Keywords: 1,25-MARRS; 1α,25(OH)2D; cytochrome P450; stomach; vitamin D deficiency; vitamin D epimers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Kuchuk N.O., Pluijm S.M.F., Van Schoor N.M., Looman C.W.N., Smit J.H., Lips P. Relationships of Serum 25-Hydroxyvitamin D to Bone Mineral Density and Serum Parathyroid Hormone and Markers of Bone Turnover in Older Persons. J. Clin. Endocrinol. Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

