Elucidating the Ordering in Self-Assembled Glycocalyx Mimicking Supramolecular Copolymers in Water
- PMID: 31387351
- PMCID: PMC6733156
- DOI: 10.1021/jacs.9b06607
Elucidating the Ordering in Self-Assembled Glycocalyx Mimicking Supramolecular Copolymers in Water
Abstract
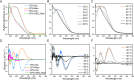
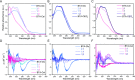
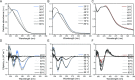
Polysaccharides present in the glycocalyx and extracellular matrix are highly important for a multitude of functions. Oligo- and polysaccharides-based biomaterials are being developed to mimic the glycocalyx, but the spatial functionalization of these polysaccharides represents a major challenge. In this paper, a series of benzene-1,3,5-tricarboxamide (BTA) based supramolecular monomers is designed and synthesized with mono- (BTA-β-d-glucose; BTA-Glc and BTA-α-d-mannose; BTA-Man) or disaccharides (BTA-β-d-cellobiose; BTA-Cel) at their periphery or a monosaccharide (BTA-OEG4-α-d-mannose; BTA-OEG4-Man) at the end of a tetraethylene glycol linker. These glycosylated BTAs have been used to generate supramolecular assemblies and it is shown that the nature of the carbohydrate appendage is crucial for the supramolecular (co)polymerization behavior. BTA-Glc and BTA-Man are shown to assemble into micrometers long 1D (bundled) fibers with opposite helicities, whereas BTA-Cel and BTA-OEG4-Man formed small spherical micelles. The latter two monomers are used in a copolymerization approach with BTA-Glc, BTA-Man, or ethylene glycol BTA (BTA-OEG4) to give 1D fibers with BTA-Cel or BTA-OEG4-Man incorporated. Consequently, the carbohydrate appendage influences both the assembly behavior and the internal order. Using this approach it is possible to create 1D-fibers with adjustable saccharide densities exhibiting tailored dynamic exchange profiles. Furthermore, hydrogels with tunable mechanical properties can be achieved, opening up possibilities for the development of multicomponent functional biomaterials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Shelke N. B.; James R.; Laurencin C. T.; Kumbar S. G. Polysaccharide Biomaterials for Drug Delivery and Regenerative Engineering. Polym. Adv. Technol. 2014, 25 (5), 448–460. 10.1002/pat.3266. - DOI
Publication types
LinkOut - more resources
Full Text Sources

