Mitochondria-hubs for regulating cellular biochemistry: emerging concepts and networks
- PMID: 31387448
- PMCID: PMC6731593
- DOI: 10.1098/rsob.190126
Mitochondria-hubs for regulating cellular biochemistry: emerging concepts and networks
Abstract
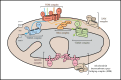
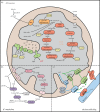
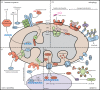
Mitochondria are iconic structures in biochemistry and cell biology, traditionally referred to as the powerhouse of the cell due to a central role in energy production. However, modern-day mitochondria are recognized as key players in eukaryotic cell biology and are known to regulate crucial cellular processes, including calcium signalling, cell metabolism and cell death, to name a few. In this review, we will discuss foundational knowledge in mitochondrial biology and provide snapshots of recent advances that showcase how mitochondrial function regulates other cellular responses.
Keywords: metabolism; mitochondria; mitochondrial biogenesis.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
