Adaptive integrate-and-fire model reproduces the dynamics of olfactory receptor neuron responses in a moth
- PMID: 31387478
- PMCID: PMC6731495
- DOI: 10.1098/rsif.2019.0246
Adaptive integrate-and-fire model reproduces the dynamics of olfactory receptor neuron responses in a moth
Abstract
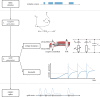
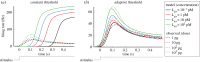
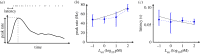
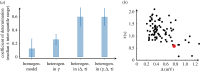
In order to understand how olfactory stimuli are encoded and processed in the brain, it is important to build a computational model for olfactory receptor neurons (ORNs). Here, we present a simple and reliable mathematical model of a moth ORN generating spikes. The model incorporates a simplified description of the chemical kinetics leading to olfactory receptor activation and action potential generation. We show that an adaptive spike threshold regulated by prior spike history is an effective mechanism for reproducing the typical phasic-tonic time course of ORN responses. Our model reproduces the response dynamics of individual neurons to a fluctuating stimulus that approximates odorant fluctuations in nature. The parameters of the spike threshold are essential for reproducing the response heterogeneity in ORNs. The model provides a valuable tool for efficient simulations of olfactory circuits.
Keywords: adaptive threshold; integrate-and-fire model; olfactory receptor neuron.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
