Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli
- PMID: 31387584
- PMCID: PMC6685171
- DOI: 10.1186/s12934-019-1177-y
Metabolic engineering for efficient supply of acetyl-CoA from different carbon sources in Escherichia coli
Abstract
Background: Acetyl-CoA is an important metabolic intermediate and serves as an acetylation precursor for the biosynthesis of various value-added acetyl-chemicals. Acetyl-CoA can be produced from glucose, acetate, or fatty acids via metabolic pathways in Escherichia coli. Although glucose is an efficient carbon source for acetyl-CoA production, the pathway from acetate to acetyl-CoA is the shortest and fatty acids can produce acetyl-CoA through fatty acid oxidation along with abundant NADH and FADH2. In this study, metabolically engineered E. coli strains for efficiently supplying acetyl-CoA from glucose, acetate, and fatty acid were constructed and applied in one-step biosynthesis of N-acetylglutamate (NAG) from glutamate and acetyl-CoA.
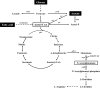
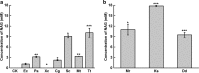
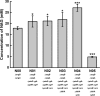
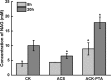
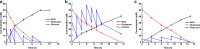
Results: A metabolically engineered E. coli strain for NAG production was constructed by overexpressing N-acetylglutamate synthase from Kitasatospora setae in E. coli BW25113 with argB and argA knockout. The strain was further engineered to utilize glucose, acetate, and fatty acid to produce acetyl-CoA. When glucose was used as a carbon source, the combined mutants of ∆ptsG::glk, ∆galR::zglf, ∆poxB::acs, ∆ldhA, and ∆pta were more efficient for supplying acetyl-CoA. The acetyl-CoA synthetase (ACS) pathway and acetate kinase-phosphate acetyltransferase (ACK-PTA) pathway from acetate to acetyl-CoA were investigated, and the ACK-PTA pathway showed to be more efficient for supplying acetyl-CoA. When fatty acid was used as a carbon source, acetyl-CoA supply was improved by deletion of fadR and constitutive expression of fadD under the strong promoter CPA1. Comparison of acetyl-CoA supply from glucose, acetate and palmitic acid revealed that a higher conversion rate of glutamate (98.2%) and productivity (an average of 6.25 mmol/L/h) were obtained when using glucose as a carbon source. The results also demonstrated the great potential of acetate and fatty acid to supply acetyl-CoA, as the molar conversion rate of glutamate was more than 80%.
Conclusions: Metabolically engineered E. coli strains were developed for NAG production. The metabolic pathways of acetyl-CoA from glucose, acetate, or fatty acid were optimized for efficient acetyl-CoA supply to enhance NAG production. The metabolic strategies for efficient acetyl-CoA supply used in this study can be exploited for other chemicals that use acetyl-CoA as a precursor or when acetylation is involved.
Keywords: Acetate; Acetyl-CoA; Fatty acid; Glucose; N-Acetylglutamate.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

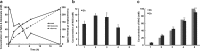
Similar articles
-
Effect of acetate formation pathway and long chain fatty acid CoA-ligase on the free fatty acid production in E. coli expressing acy-ACP thioesterase from Ricinus communis.Metab Eng. 2012 Jul;14(4):380-7. doi: 10.1016/j.ymben.2012.03.007. Epub 2012 Mar 30. Metab Eng. 2012. PMID: 22480945
-
Characterizing the effect of expression of an acetyl-CoA synthetase insensitive to acetylation on co-utilization of glucose and acetate in batch and continuous cultures of E. coli W.Microb Cell Fact. 2018 Jul 9;17(1):109. doi: 10.1186/s12934-018-0955-2. Microb Cell Fact. 2018. PMID: 29986728 Free PMC article.
-
Eliminating acetate formation improves citramalate production by metabolically engineered Escherichia coli.Microb Cell Fact. 2017 Jun 21;16(1):114. doi: 10.1186/s12934-017-0729-2. Microb Cell Fact. 2017. PMID: 28637476 Free PMC article.
-
Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing.Metab Eng. 2016 Jul;36:99-115. doi: 10.1016/j.ymben.2016.03.006. Epub 2016 Mar 23. Metab Eng. 2016. PMID: 27016336 Review.
-
Strategies for optimizing acetyl-CoA formation from glucose in bacteria.Trends Biotechnol. 2022 Feb;40(2):149-165. doi: 10.1016/j.tibtech.2021.04.004. Epub 2021 May 5. Trends Biotechnol. 2022. PMID: 33965247 Review.
Cited by
-
Microbial Cell Factory of Baccatin III Preparation in Escherichia coli by Increasing DBAT Thermostability and in vivo Acetyl-CoA Supply.Front Microbiol. 2022 Jan 12;12:803490. doi: 10.3389/fmicb.2021.803490. eCollection 2021. Front Microbiol. 2022. PMID: 35095813 Free PMC article.
-
Metabolic engineering of Escherichia coli for the utilization of ethanol.J Biol Res (Thessalon). 2020 Jan 21;27:1. doi: 10.1186/s40709-020-0111-0. eCollection 2020 Dec. J Biol Res (Thessalon). 2020. PMID: 31993378 Free PMC article.
-
Microbial Upgrading of Acetate into Value-Added Products-Examining Microbial Diversity, Bioenergetic Constraints and Metabolic Engineering Approaches.Int J Mol Sci. 2020 Nov 20;21(22):8777. doi: 10.3390/ijms21228777. Int J Mol Sci. 2020. PMID: 33233586 Free PMC article. Review.
-
A genetically encoded fluorescent biosensor for visualization of acetyl-CoA in live cells.Cell Chem Biol. 2025 Feb 20;32(2):325-337.e10. doi: 10.1016/j.chembiol.2025.01.002. Epub 2025 Jan 27. Cell Chem Biol. 2025. PMID: 39874963
-
Design and Characterization of an Osmotic Pump System for Optimal Feeding and pH Control in E. coli Culture to Increase Biomass.Iran J Pharm Res. 2024 Feb 17;23(1):e138677. doi: 10.5812/ijpr-138677. eCollection 2024 Jan-Dec. Iran J Pharm Res. 2024. PMID: 39005735 Free PMC article.
References
-
- Kildegaard KR, Jensen NB, Schneider K, Czarnotta E, Ozdemir E, Klein T, Maury J, Ebert BE, Christensen HB, Chen Y, et al. Engineering and systems-level analysis of Saccharomyces cerevisiae for production of 3-hydroxypropionic acid via malonyl-CoA reductase-dependent pathway. Microb Cell Fact. 2016;15:53. doi: 10.1186/s12934-016-0451-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

