Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy
- PMID: 31387880
- PMCID: PMC6693002
- DOI: 10.1182/bloodadvances.2019000219
Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy
Abstract
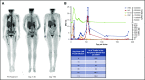
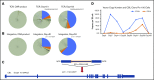
Reexpansion of CAR T cells led to further investigations which confirmed the clonal nature of this expansion.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

