Neuregulin Signaling Is a Mechanism of Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma
- PMID: 31387891
- PMCID: PMC6825559
- DOI: 10.1158/1535-7163.MCT-19-0163
Neuregulin Signaling Is a Mechanism of Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma
Abstract
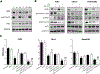
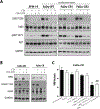
EGFR signaling confers resistance to radiotherapy and is a validated target in head and neck squamous cell carcinoma (HNSCC). The inhibition of EGFR in combination with radiotherapy improves local control and overall survival in these patients; however, therapeutic resistance limits the efficacy of this approach. We therefore sought to identify cellular mechanisms that cause resistance to EGFR inhibition and radiotherapy in HNSCC. Though clonal isolation of carcinoma cells exposed to increasing concentrations of cetuximab, we found that resistant cells upregulate prosurvival ErbB3 and AKT signaling. Using EFM-19 cells and confirmatory analysis of protein levels, we demonstrate that cetuximab resistance is characterized by enhanced neuregulin expression identifying a novel adaptive mechanism of therapeutic resistance. Inhibition of this autocrine loop with CDX-3379 (an ErbB3 specific antibody) was sufficient to block ErbB3/AKT signaling in cetuximab resistant cells. The combination of CDX-3379 and cetuximab reduced proliferation and survival after radiotherapy in several HNSCC cell lines. These in vitro findings were confirmed in xenograft tumor growth experiments including an approach using growth factor-supplemented Matrigel. In vivo, the delivery of EGFR and ErbB3 antibodies significantly reduced tumor growth in cetuximab-resistant FaDu and CAL27 xenografts. In summary, this work demonstrates that autocrine NRG ligand secretion is a mechanism for therapeutic resistance to cetuximab and radiotherapy. This cross-resistance to both therapeutic modalities identifies NRG as an actionable therapeutic target for improving treatment regimens in HNSCC.
©2019 American Association for Cancer Research.
Figures




References
-
- Contessa JN, Abell A, Valerie K, Lin PS, Schmidt-Ullrich RK. ErbB receptor tyrosine kinase network inhibition radiosensitizes carcinoma cells. Int J Radiat Oncol Biol Phys. 2006;65:851–8. - PubMed
-
- Ang KK, Andratschke NH, Milas L. Epidermal growth factor receptor and response of head-and-neck carcinoma to therapy. Int J Radiat Oncol Biol Phys. 2004;58:959–65. - PubMed
-
- Milas L, Mason KA, Ang KK. Epidermal growth factor receptor and its inhibition in radiotherapy: in vivo findings. Int J Radiat Biol. 2003;79:539–45. - PubMed
-
- Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

