Anopheles Salivary Gland Architecture Shapes Plasmodium Sporozoite Availability for Transmission
- PMID: 31387905
- PMCID: PMC6686039
- DOI: 10.1128/mBio.01238-19
Anopheles Salivary Gland Architecture Shapes Plasmodium Sporozoite Availability for Transmission
Abstract
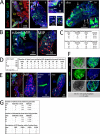
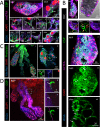
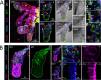
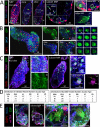
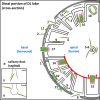
Plasmodium sporozoites (SPZs) must traverse the mosquito salivary glands (SGs) to reach a new vertebrate host and continue the malaria disease cycle. Although SGs can harbor thousands of sporozoites, only 10 to 100 are deposited into a host during probing. To determine how the SGs might function as a bottleneck in SPZ transmission, we have characterized Anopheles stephensi SGs infected with the rodent malaria parasite Plasmodium berghei using immunofluorescence confocal microscopy. Our analyses corroborate findings from previous electron microscopy studies and provide new insights into the invasion process. We identified sites of SPZ accumulation within SGs across a range of infection intensities. Although SPZs were most often seen in the distal lateral SG lobes, they were also observed in the medial and proximal lateral lobes. Most parasites were associated with either the basement membrane or secretory cavities. SPZs accumulated at physical barriers, including fused salivary ducts and extensions of the chitinous salivary duct wall into the distal lumen. SPZs were observed only rarely within salivary ducts. SPZs appeared to contact each other in many different quantities, not just in the previously described large bundles. Within parasite bundles, all of the SPZs were oriented in the same direction. We found that moderate levels of infection did not necessarily correlate with major SG disruptions or abundant SG cell death. Altogether, our findings suggest that SG architecture largely acts as a barrier to SPZ transmission.IMPORTANCE Malaria continues to have a devastating impact on human health. With growing resistance to insecticides and antimalarial drugs, as well as climate change predictions indicating expansion of vector territories, the impact of malaria is likely to increase. Additional insights regarding pathogen migration through vector mosquitoes are needed to develop novel methods to prevent transmission to new hosts. Pathogens, including the microbes that cause malaria, must invade the salivary glands (SGs) for transmission. Since SG traversal is required for parasite transmission, SGs are ideal targets for transmission-blocking strategies. The work presented here highlights the role that mosquito SG architecture plays in limiting parasite traversal, revealing how the SG transmission bottleneck is imposed. Further, our data provide unprecedented detail about SG-sporozoite interactions and gland-to-gland variation not provided in previous studies.
Keywords: malaria; mosquito; salivary gland; sporozoite.
Copyright © 2019 Wells and Andrew.
Figures


References
-
- World Health Organization. 2017. World malaria report 2017. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
