Disruption of Glycolysis by Nutritional Immunity Activates a Two-Component System That Coordinates a Metabolic and Antihost Response by Staphylococcus aureus
- PMID: 31387906
- PMCID: PMC6686040
- DOI: 10.1128/mBio.01321-19
Disruption of Glycolysis by Nutritional Immunity Activates a Two-Component System That Coordinates a Metabolic and Antihost Response by Staphylococcus aureus
Abstract
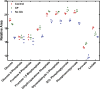
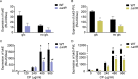
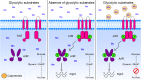
During infection, bacteria use two-component signal transduction systems to sense and adapt to the dynamic host environment. Despite critically contributing to infection, the activating signals of most of these regulators remain unknown. This also applies to the Staphylococcus aureus ArlRS two-component system, which contributes to virulence by coordinating the production of toxins, adhesins, and a metabolic response that enables the bacterium to overcome host-imposed manganese starvation. Restricting the availability of essential transition metals, a strategy known as nutritional immunity, constitutes a critical defense against infection. In this work, expression analysis revealed that manganese starvation imposed by the immune effector calprotectin or by the absence of glycolytic substrates activates ArlRS. Manganese starvation imposed by calprotectin also activated the ArlRS system even when glycolytic substrates were present. A combination of metabolomics, mutational analysis, and metabolic feeding experiments revealed that ArlRS is activated by alterations in metabolic flux occurring in the latter half of the glycolytic pathway. Moreover, calprotectin was found to induce expression of staphylococcal leukocidins in an ArlRS-dependent manner. These studies indicated that ArlRS is a metabolic sensor that allows S. aureus to integrate multiple environmental stresses that alter glycolytic flux to coordinate an antihost response and to adapt to manganese starvation. They also established that the latter half of glycolysis represents a checkpoint to monitor metabolic state in S. aureus Altogether, these findings contribute to understanding how invading pathogens, such as S. aureus, adapt to the host during infection and suggest the existence of similar mechanisms in other bacterial species.IMPORTANCE Two-component regulatory systems enable bacteria to adapt to changes in their environment during infection by altering gene expression and coordinating antihost responses. Despite the critical role of two-component systems in bacterial survival and pathogenesis, the activating signals for most of these regulators remain unidentified. This is exemplified by ArlRS, a Staphylococcus aureus global regulator that contributes to virulence and to resisting host-mediated restriction of essential nutrients, such as manganese. In this report, we demonstrate that manganese starvation and the absence of glycolytic substrates activate ArlRS. Further investigations revealed that ArlRS is activated when the latter half of glycolysis is disrupted, suggesting that S. aureus monitors flux through the second half of this pathway. Host-imposed manganese starvation also induced the expression of pore-forming toxins in an ArlRS-dependent manner. Cumulatively, this work reveals that ArlRS acts as a sensor that links nutritional status, cellular metabolism, and virulence regulation.
Keywords: ArlRS; Staphylococcus aureus; calprotectin; glycolysis; manganese; nutritional immunity.
Copyright © 2019 Párraga Solórzano et al.
Figures
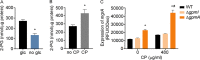
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

