A Chaperone for the Stator Units of a Bacterial Flagellum
- PMID: 31387912
- PMCID: PMC6686046
- DOI: 10.1128/mBio.01732-19
A Chaperone for the Stator Units of a Bacterial Flagellum
Abstract
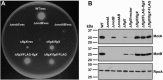
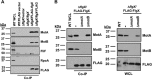
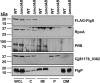
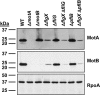
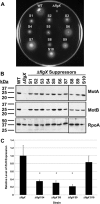
The stator units of the flagellum supply power to the flagellar motor via ion transport across the cytoplasmic membrane and generate torque on the rotor for rotation. Flagellar motors across bacterial species have evolved adaptations that impact and enhance stator function to meet the demands of each species, including producing stator units using different fuel types or various stator units for different motility modalities. Campylobacter jejuni produces one of the most complex and powerful flagellar motors by positioning 17 stator units at a greater radial distance than in most other bacteria to increase power and torque for high velocity of motility. We report another evolutionary adaptation impacting flagellar stators by identifying FlgX as a chaperone for C. jejuni stator units to ensure sufficient power and torque for flagellar rotation and motility. We discovered that FlgX maintains MotA and MotB stator protein integrity likely through a direct interaction with MotA that prevents their degradation. Suppressor analysis suggested that the physiology of C. jejuni drives the requirement for FlgX to protect stator units from proteolysis by the FtsH protease complex. C. jejuni ΔflgX was strongly attenuated for colonization of the natural avian host, but colonization capacity was greatly restored by a single mutation in MotA. These findings suggest that the likely sole function of FlgX is to preserve stator unit integrity for the motility required for host interactions. Our findings demonstrate another evolved adaptation in motile bacteria to ensure the equipment of the flagellar motor with sufficient power to generate torque for motility.IMPORTANCE The bacterial flagellum is a reversible rotating motor powered by ion transport through stator units, which also exert torque on the rotor component to turn the flagellum for motility. Species-specific adaptations to flagellar motors impact stator function to meet the demands of each species to sufficiently power flagellar rotation. We identified another evolutionary adaptation by discovering that FlgX of Campylobacter jejuni preserves the integrity of stator units by functioning as a chaperone to protect stator proteins from degradation by the FtsH protease complex due to the physiology of the bacterium. FlgX is required to maintain a level of stator units sufficient to power the naturally high-torque flagellar motor of C. jejuni for motility in intestinal mucosal layers to colonize hosts. Our work continues to identify an increasing number of adaptations to flagellar motors across bacterial species that provide the mechanics necessary for producing an effective rotating nanomachine for motility.
Keywords: Campylobacter jejuni; FlgX; MotA; MotB; chaperone; flagellar motility; stator.
Copyright © 2019 Ribardo et al.
Figures


Comment in
-
Time for a motor check.Nat Rev Microbiol. 2019 Oct;17(10):587. doi: 10.1038/s41579-019-0262-x. Nat Rev Microbiol. 2019. PMID: 31435027 No abstract available.
Similar articles
-
Tetrameric PilZ protein stabilizes stator ring in complex flagellar motor and is required for motility in Campylobacter jejuni.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2412594121. doi: 10.1073/pnas.2412594121. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793078 Free PMC article.
-
Effect of the MotA(M206I) Mutation on Torque Generation and Stator Assembly in the Salmonella H+-Driven Flagellar Motor.J Bacteriol. 2019 Feb 25;201(6):e00727-18. doi: 10.1128/JB.00727-18. Print 2019 Mar 15. J Bacteriol. 2019. PMID: 30642987 Free PMC article.
-
Structure and Function of Stator Units of the Bacterial Flagellar Motor.Cell. 2020 Oct 1;183(1):244-257.e16. doi: 10.1016/j.cell.2020.08.016. Epub 2020 Sep 14. Cell. 2020. PMID: 32931735
-
Dynamism and regulation of the stator, the energy conversion complex of the bacterial flagellar motor.Curr Opin Microbiol. 2015 Dec;28:66-71. doi: 10.1016/j.mib.2015.07.015. Epub 2015 Oct 23. Curr Opin Microbiol. 2015. PMID: 26457925 Review.
-
Evolution of the Stator Elements of Rotary Prokaryote Motors.J Bacteriol. 2020 Jan 15;202(3):e00557-19. doi: 10.1128/JB.00557-19. Print 2020 Jan 15. J Bacteriol. 2020. PMID: 31591272 Free PMC article. Review.
Cited by
-
Role of DegQ in differential stability of flagellin subunits in Vibrio vulnificus.NPJ Biofilms Microbiomes. 2021 Apr 8;7(1):32. doi: 10.1038/s41522-021-00206-7. NPJ Biofilms Microbiomes. 2021. PMID: 33833236 Free PMC article.
-
FliO is an evolutionarily conserved yet diversified core component of the bacterial flagellar type III secretion system.bioRxiv [Preprint]. 2025 May 6:2025.05.06.652439. doi: 10.1101/2025.05.06.652439. bioRxiv. 2025. PMID: 40654941 Free PMC article. Preprint.
-
The evolutionary path of chemosensory and flagellar macromolecular machines in Campylobacterota.PLoS Genet. 2022 Jul 14;18(7):e1010316. doi: 10.1371/journal.pgen.1010316. eCollection 2022 Jul. PLoS Genet. 2022. PMID: 35834583 Free PMC article.
-
Tetrameric PilZ protein stabilizes stator ring in complex flagellar motor and is required for motility in Campylobacter jejuni.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2412594121. doi: 10.1073/pnas.2412594121. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793078 Free PMC article.
-
Time for a motor check.Nat Rev Microbiol. 2019 Oct;17(10):587. doi: 10.1038/s41579-019-0262-x. Nat Rev Microbiol. 2019. PMID: 31435027 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

