Efficient Inhibition of Human Papillomavirus Infection by L2 Minor Capsid-Derived Lipopeptide
- PMID: 31387913
- PMCID: PMC6686047
- DOI: 10.1128/mBio.01834-19
Efficient Inhibition of Human Papillomavirus Infection by L2 Minor Capsid-Derived Lipopeptide
Abstract
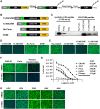
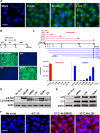
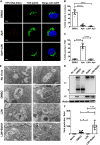
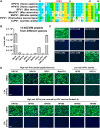
The amino (N)-terminal region of human papillomavirus (HPV) minor capsid protein (L2) is a highly conserved region which is essential for establishing viral infection. Despite its importance in viral infectivity, the role of the HPV N-terminal domain has yet to be fully characterized. Using fine mapping analysis, we identified a 36-amino-acid (aa) peptide sequence of the L2 N terminus, termed L2N, that is critical for HPV infection. Ectopic expression of L2N with the transmembrane sequence on the target cell surface conferred resistance to HPV infection. Additionally, L2N peptide with chemical or enzymatic lipidation at the carboxyl (C) terminus efficiently abrogated HPV infection in target cells. Among the synthetic L2N lipopeptides, a stearoylated lipopeptide spanning aa 13 to 46 (13-46st) exhibited the most potent anti-HPV activity, with a half-maximal inhibitory concentration (IC50) of ∼200 pM. Furthermore, we demonstrated that the 13-46st lipopeptide inhibited HPV entry by blocking trans-Golgi network retrograde trafficking of virion particles, leading to rapid degradation. Fundamentally, the inhibitory effect of L2N lipopeptides appeared to be evolutionarily conserved, as they showed cross-type inhibition among various papillomaviruses. In conclusion, our findings provide new insights into the critical role of the L2N sequence in the HPV entry mechanism and identify the therapeutic potential of L2N lipopeptide as an effective anti-HPV agent.IMPORTANCE HPV is a human oncogenic virus that causes a major public health problem worldwide, which is responsible for approximately 5% of total human cancers and almost all cases of cervical cancers. HPV capsid consists of two structure proteins, the major capsid L1 protein and the minor capsid L2 protein. While L2 plays critical roles during the viral life cycle, the molecular mechanism in viral entry remains elusive. Here, we performed fine mapping of the L2 N-terminal region and defined a short 36-amino-acid peptide, called L2N, which is critical for HPV infection. Specifically, L2N peptide with carboxyl-terminal lipidation acted as a potent and cross-type HPV inhibitor. Taken together, data from our study highlight the essential role of the L2N sequence at the early step of HPV entry and suggests the L2N lipopeptide as a new strategy to broadly prevent HPV infection.
Keywords: L2N lipopeptide; entry inhibitor; human papillomavirus; minor capsid protein.
Copyright © 2019 Yan et al.
Figures



References
-
- Tachezy R, Rector A, Havelkova M, Wollants E, Fiten P, Opdenakker G, Jenson B, Sundberg J, Van Ranst M. 2002. Avian papillomaviruses: the parrot Psittacus erithacus papillomavirus (PePV) genome has a unique organization of the early protein region and is phylogenetically related to the chaffinch papillomavirus. BMC Microbiol 2:19. doi: 10.1186/1471-2180-2-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
