α-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes
- PMID: 31387941
- PMCID: PMC6719451
- DOI: 10.1083/jcb.201903124
α-Integrins dictate distinct modes of type IV collagen recruitment to basement membranes
Abstract
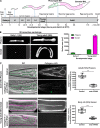
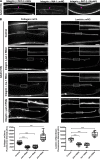
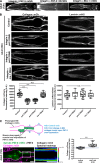
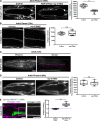
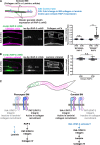
Basement membranes (BMs) are cell-associated extracellular matrices that support tissue integrity, signaling, and barrier properties. Type IV collagen is critical for BM function, yet how it is directed into BMs in vivo is unclear. Through live-cell imaging of endogenous localization, conditional knockdown, and misexpression experiments, we uncovered distinct mechanisms of integrin-mediated collagen recruitment to Caenorhabditis elegans postembryonic gonadal and pharyngeal BMs. The putative laminin-binding αINA-1/βPAT-3 integrin was selectively activated in the gonad and recruited laminin, which directed moderate collagen incorporation. In contrast, the putative Arg-Gly-Asp (RGD)-binding αPAT-2/βPAT-3 integrin was activated in the pharynx and recruited high levels of collagen in an apparently laminin-independent manner. Through an RNAi screen, we further identified the small GTPase RAP-3 (Rap1) as a pharyngeal-specific PAT-2/PAT-3 activator that modulates collagen levels. Together, these studies demonstrate that tissues can use distinct mechanisms to direct collagen incorporation into BMs to precisely control collagen levels and construct diverse BMs.
© 2019 Jayadev et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

