The LRRC8/VRAC anion channel facilitates myogenic differentiation of murine myoblasts by promoting membrane hyperpolarization
- PMID: 31387946
- PMCID: PMC6768655
- DOI: 10.1074/jbc.RA119.008840
The LRRC8/VRAC anion channel facilitates myogenic differentiation of murine myoblasts by promoting membrane hyperpolarization
Abstract
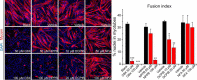
Skeletal muscle myoblast differentiation involves elaborate signaling networks, including the activity of various ion channels and transporters. Several K+ and Ca2+ channels have been shown to affect myogenesis, but little is known about roles of Cl- channels in the associated processes. Here, we report that the leucine-rich repeat containing family 8 (LRRC8)/volume-regulated anion channel (VRAC) promotes mouse myoblast differentiation. All LRRC8 subunits of heteromeric VRAC were expressed during myotube formation of murine C2C12 myoblasts. Pharmacological VRAC inhibitors, siRNA-mediated knockdown of the essential VRAC subunit LRRC8A, or VRAC activity-suppressing overexpression of LRRC8A effectively reduced the expression of the myogenic transcription factor myogenin and suppressed myoblast fusion while not affecting myoblast proliferation. We found that inhibiting VRAC impairs plasma membrane hyperpolarization early during differentiation. At later times (more than 6 h after inducing differentiation), VRAC inhibition no longer suppressed myoblast differentiation, suggesting that VRAC acts upstream of K+ channel activation. Consequently, VRAC inhibition prevented the increase of intracellular steady-state Ca2+ levels that normally occurs during myogenesis. Our results may explain the mechanism for the thinning of skeletal muscle bundles observed in LRRC8A-deficient mice and highlight the importance of the LRRC8/VRAC anion channel in cell differentiation.
Keywords: C2C12 myoblasts; calcium; cell differentiation; chloride channel; hyperpolarization; membrane potential; myogenesis; skeletal muscle; volume-regulated anion channel (VRAC).
© 2019 Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
LRRC8 channel activation and reduction in cytosolic chloride concentration during early differentiation of C2C12 myoblasts.Biochem Biophys Res Commun. 2020 Nov 12;532(3):482-488. doi: 10.1016/j.bbrc.2020.08.080. Epub 2020 Sep 4. Biochem Biophys Res Commun. 2020. PMID: 32892951
-
Intracellular and extracellular loops of LRRC8 are essential for volume-regulated anion channel function.J Gen Physiol. 2018 Jul 2;150(7):1003-1015. doi: 10.1085/jgp.201812016. Epub 2018 May 31. J Gen Physiol. 2018. PMID: 29853476 Free PMC article.
-
Absolute Protein Amounts and Relative Abundance of Volume-regulated Anion Channel (VRAC) LRRC8 Subunits in Cells and Tissues Revealed by Quantitative Immunoblotting.Int J Mol Sci. 2019 Nov 23;20(23):5879. doi: 10.3390/ijms20235879. Int J Mol Sci. 2019. PMID: 31771171 Free PMC article.
-
The identification of a volume-regulated anion channel: an amazing Odyssey.Acta Physiol (Oxf). 2015 Apr;213(4):868-81. doi: 10.1111/apha.12450. Epub 2015 Jan 28. Acta Physiol (Oxf). 2015. PMID: 25565132 Review.
-
VRAC: molecular identification as LRRC8 heteromers with differential functions.Pflugers Arch. 2016 Mar;468(3):385-93. doi: 10.1007/s00424-015-1766-5. Epub 2015 Dec 3. Pflugers Arch. 2016. PMID: 26635246 Review.
Cited by
-
Measuring Absolute Membrane Potential Across Space and Time.Annu Rev Biophys. 2021 May 6;50:447-468. doi: 10.1146/annurev-biophys-062920-063555. Epub 2021 Mar 2. Annu Rev Biophys. 2021. PMID: 33651949 Free PMC article. Review.
-
SWELL1 regulates skeletal muscle cell size, intracellular signaling, adiposity and glucose metabolism.Elife. 2020 Sep 15;9:e58941. doi: 10.7554/eLife.58941. Elife. 2020. PMID: 32930093 Free PMC article.
-
Fine Tuning of Calcium Constitutive Entry by Optogenetically-Controlled Membrane Polarization: Impact on Cell Migration.Cells. 2020 Jul 13;9(7):1684. doi: 10.3390/cells9071684. Cells. 2020. PMID: 32668787 Free PMC article.
-
Approach for Elucidating the Molecular Mechanism of Epithelial to Mesenchymal Transition in Fibrosis of Asthmatic Airway Remodeling Focusing on Cl- Channels.Int J Mol Sci. 2023 Dec 25;25(1):289. doi: 10.3390/ijms25010289. Int J Mol Sci. 2023. PMID: 38203460 Free PMC article. Review.
-
Sphingosine-1-phosphate activates LRRC8 volume-regulated anion channels through Gβγ signalling.J Physiol. 2025 Aug;603(15):4329-4344. doi: 10.1113/JP286665. Epub 2024 Nov 4. J Physiol. 2025. PMID: 39496493 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

