Application of millisecond time-resolved solid state NMR to the kinetics and mechanism of melittin self-assembly
- PMID: 31387974
- PMCID: PMC6708326
- DOI: 10.1073/pnas.1908006116
Application of millisecond time-resolved solid state NMR to the kinetics and mechanism of melittin self-assembly
Abstract
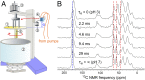
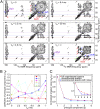
Common experimental approaches for characterizing structural conversion processes such as protein folding and self-assembly do not report on all aspects of the evolution from an initial state to the final state. Here, we demonstrate an approach that is based on rapid mixing, freeze-trapping, and low-temperature solid-state NMR (ssNMR) with signal enhancements from dynamic nuclear polarization (DNP). Experiments on the folding and tetramerization of the 26-residue peptide melittin following a rapid pH jump show that multiple aspects of molecular structure can be followed with millisecond time resolution, including secondary structure at specific isotopically labeled sites, intramolecular and intermolecular contacts between specific pairs of labeled residues, and overall structural order. DNP-enhanced ssNMR data reveal that conversion of conformationally disordered melittin monomers at low pH to α-helical conformations at neutral pH occurs on nearly the same timescale as formation of antiparallel melittin dimers, about 6 to 9 ms for 0.3 mM melittin at 24 °C in aqueous solution containing 20% (vol/vol) glycerol and 75 mM sodium phosphate. Although stopped-flow fluorescence data suggest that melittin tetramers form quickly after dimerization, ssNMR spectra show that full structural order within melittin tetramers develops more slowly, in ∼60 ms. Time-resolved ssNMR is likely to find many applications to biomolecular structural conversion processes, including early stages of amyloid formation, viral capsid formation, and protein-protein recognition.
Keywords: melittin; protein folding; self-assembly; solid-state NMR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Royer C. A., Probing protein folding and conformational transitions with fluorescence. Chem. Rev. 106, 1769–1784 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

