Rhodium-catalysed direct hydroarylation of alkenes and alkynes with phosphines through phosphorous-assisted C-H activation
- PMID: 31387999
- PMCID: PMC6684548
- DOI: 10.1038/s41467-019-11420-5
Rhodium-catalysed direct hydroarylation of alkenes and alkynes with phosphines through phosphorous-assisted C-H activation
Abstract
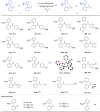
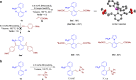
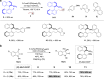
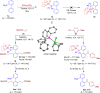
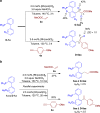
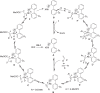
Biarylphosphines have been widely applied as ligands in various synthetic methods, especially in transition-metal-catalysed carbon-carbon and carbon-heteroatom bond cross-coupling reactions. Based on the outstanding properties of the parent scaffolds, a general method for in situ modification of the commercial tertiary phosphine ligands to access a series of ligands is in high demand. Here we show that a rhodium-catalysed system is introduced for the hydroarylation of alkenes and alkynes with tertiary phosphines through P(III)-chelation assisted C-H activation. A series of ligand libraries containing alkyl and alkenyl substituted groups with different steric and electronic properties are obtained in high yields. Furthermore, several experimental studies are performed to uncover the key mechanistic features of the linear-selective hydroarylation of alkenes and branch-selective hydroarylation of alkynes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

