Hyperosmolar environment and intestinal epithelial cells: impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro
- PMID: 31388052
- PMCID: PMC6684637
- DOI: 10.1038/s41598-019-47851-9
Hyperosmolar environment and intestinal epithelial cells: impact on mitochondrial oxygen consumption, proliferation, and barrier function in vitro
Abstract
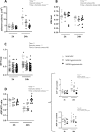
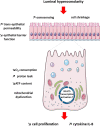
The aim of the present study was to elucidate the in vitro short-term (2-h) and longer-term (24-h) effects of hyperosmolar media (500 and 680 mOsm/L) on intestinal epithelial cells using the human colonocyte Caco-2 cell line model. We found that a hyperosmolar environment slowed down cell proliferation compared to normal osmolarity (336 mOsm/L) without inducing cell detachment or necrosis. This was associated with a transient reduction of cell mitochondrial oxygen consumption, increase in proton leak, and decrease in intracellular ATP content. The barrier function of Caco-2 monolayers was also transiently affected since increased paracellular apical-to-basal permeability and modified electrolyte permeability were measured, allowing partial equilibration of the trans-epithelial osmotic difference. In addition, hyperosmotic stress induced secretion of the pro-inflammatory cytokine IL-8. By measuring expression of genes involved in energy metabolism, tight junction forming, electrolyte permeability and intracellular signaling, different response patterns to hyperosmotic stress occurred depending on its intensity and duration. These data highlight the potential impact of increased luminal osmolarity on the intestinal epithelium renewal and barrier function and point out some cellular adaptive capacities towards luminal hyperosmolar environment.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Alpha-Melanocyte Stimulating Hormone Protects against Cytokine-Induced Barrier Damage in Caco-2 Intestinal Epithelial Monolayers.PLoS One. 2017 Jan 19;12(1):e0170537. doi: 10.1371/journal.pone.0170537. eCollection 2017. PLoS One. 2017. PMID: 28103316 Free PMC article.
-
Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model.PLoS One. 2016 Aug 23;11(8):e0161635. doi: 10.1371/journal.pone.0161635. eCollection 2016. PLoS One. 2016. PMID: 27551722 Free PMC article.
-
miR-200b inhibits TNF-α-induced IL-8 secretion and tight junction disruption of intestinal epithelial cells in vitro.Am J Physiol Gastrointest Liver Physiol. 2017 Feb 1;312(2):G123-G132. doi: 10.1152/ajpgi.00316.2016. Epub 2016 Dec 15. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 27979826
-
Myrrh exerts barrier-stabilising and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells.Int J Colorectal Dis. 2017 May;32(5):623-634. doi: 10.1007/s00384-016-2736-x. Epub 2016 Dec 15. Int J Colorectal Dis. 2017. PMID: 27981377
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells.Foods. 2021 Jul 15;10(7):1634. doi: 10.3390/foods10071634. Foods. 2021. PMID: 34359504 Free PMC article.
-
Effects of continuously infusing glucose or casein into the terminal ileum on biomarkers of metabolism, inflammation, and intestinal morphology in growing pigs.J Anim Sci. 2021 Jul 1;99(7):skab163. doi: 10.1093/jas/skab163. J Anim Sci. 2021. PMID: 34015122 Free PMC article.
-
Hypo-osmotic stress induces the epithelial alarmin IL-33 in the colonic barrier of ulcerative colitis.Sci Rep. 2022 Jul 7;12(1):11550. doi: 10.1038/s41598-022-15573-0. Sci Rep. 2022. PMID: 35798804 Free PMC article.
-
Fate of undigested proteins in the pig large intestine: What impact on the colon epithelium?Anim Nutr. 2021 Sep 17;9:110-118. doi: 10.1016/j.aninu.2021.08.001. eCollection 2022 Jun. Anim Nutr. 2021. PMID: 35573094 Free PMC article. Review.
-
Dietary supplementation with inosine-5'-monophosphate improves the functional, energetic, and antioxidant status of liver and muscle growth in pigs.Sci Rep. 2022 Jan 10;12(1):350. doi: 10.1038/s41598-021-04023-y. Sci Rep. 2022. PMID: 35013384 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

