Transcriptomic analysis of insecticide resistance in the lymphatic filariasis vector Culex quinquefasciatus
- PMID: 31388075
- PMCID: PMC6684662
- DOI: 10.1038/s41598-019-47850-w
Transcriptomic analysis of insecticide resistance in the lymphatic filariasis vector Culex quinquefasciatus
Abstract
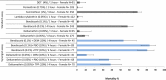
Culex quinquefasciatus plays an important role in transmission of vector-borne diseases of public health importance, including lymphatic filariasis (LF), as well as many arboviral diseases. Currently, efforts to tackle C. quinquefasciatus vectored diseases are based on either mass drug administration (MDA) for LF, or insecticide-based interventions. Widespread and intensive insecticide usage has resulted in increased resistance in mosquito vectors, including C. quinquefasciatus. Herein, the transcriptome profile of Ugandan bendiocarb-resistant C. quinquefasciatus was explored to identify candidate genes associated with insecticide resistance. High levels of insecticide resistance were observed for five out of six insecticides tested, with the lowest mortality (0.97%) reported to permethrin, while for DDT, lambdacyhalothrin, bendiocarb and deltamethrin the mortality rate ranged from 1.63-3.29%. Resistance to bendiocarb in exposed mosquitoes was marked, with 2.04% mortality following 1 h exposure and 58.02% after 4 h. Genotyping of the G119S Ace-1 target site mutation detected a highly significant association (p < 0.0001; OR = 25) between resistance and Ace1-119S. However, synergist assays using the P450 inhibitor PBO, or the esterase inhibitor TPP resulted in markedly increased mortality (to ≈80%), suggesting a role of metabolic resistance in the resistance phenotype. Using a novel, custom 60 K whole-transcriptome microarray 16 genes significantly overexpressed in resistant mosquitoes were detected, with the P450 Cyp6z18 showing the highest differential gene expression (>8-fold increase vs unexposed controls). These results provide evidence that bendiocarb resistance in Ugandan C. quinquefasciatus is mediated by both target-site mechanisms and over-expression of detoxification enzymes.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

