Convolutional Neural Networks for Spectroscopic Analysis in Retinal Oximetry
- PMID: 31388136
- PMCID: PMC6684811
- DOI: 10.1038/s41598-019-47621-7
Convolutional Neural Networks for Spectroscopic Analysis in Retinal Oximetry
Abstract
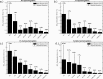
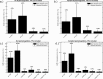
Retinal oximetry is a non-invasive technique to investigate the hemodynamics, vasculature and health of the eye. Current techniques for retinal oximetry have been plagued by quantitatively inconsistent measurements and this has greatly limited their adoption in clinical environments. To become clinically relevant oximetry measurements must become reliable and reproducible across studies and locations. To this end, we have developed a convolutional neural network algorithm for multi-wavelength oximetry, showing a greatly improved calculation performance in comparison to previously reported techniques. The algorithm is calibration free, performs sensing of the four main hemoglobin conformations with no prior knowledge of their characteristic absorption spectra and, due to the convolution-based calculation, is invariable to spectral shifting. We show, herein, the dramatic performance improvements in using this algorithm to deduce effective oxygenation (SO2), as well as the added functionality to accurately measure fractional oxygenation ([Formula: see text]). Furthermore, this report compares, for the first time, the relative performance of several previously reported multi-wavelength oximetry algorithms in the face of controlled spectral variations. The improved ability of the algorithm to accurately and independently measure hemoglobin concentrations offers a high potential tool for disease diagnosis and monitoring when applied to retinal spectroscopy.
Conflict of interest statement
Dominic Sauvageau is part owner of Zilia, Inc., which partially funded this research and supplied
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

