Effect of medical thoracoscopy-guided intrapleural docetaxel therapy to manage malignant pleural effusion in patients with non-small cell lung cancer: A pilot study
- PMID: 31389192
- PMCID: PMC6775018
- DOI: 10.1111/1759-7714.13158
Effect of medical thoracoscopy-guided intrapleural docetaxel therapy to manage malignant pleural effusion in patients with non-small cell lung cancer: A pilot study
Abstract
Background: Although chemical pleurodesis is a useful treatment option for malignant pleural effusion, little is known about the effects of intrapleural docetaxel therapy.
Objectives: This study aimed to evaluate the effects of medical thoracoscopy-guided intrapleural docetaxel therapy in patients with lung cancer.
Methods: Patients with lung cancer who diagnosed malignant pleural effusion were enrolled in this single-center prospective pilot study. The clinical response and toxicity were evaluated at two, six and 12 weeks post-treatment.
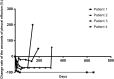
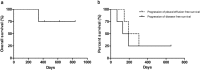
Results: Medical thoracoscopy-guided intrapleural docetaxel therapy was conducted in four patients between June 2016 and August 2017. The control rate of malignant pleural effusion was 100% (4/4), and the progression-free duration of effusion was 527 ± 109 days. No serious adverse events were observed, but only mild-to-moderate adverse events were observed and well controlled by conservative management. Although the overall quality of life assessed using questionnaires did not show significant improvement, symptom burden due to dyspnea was significantly improved.
Conclusions: Intrapleural docetaxel therapy with medical thoracoscopy showed good clinical responses, relieving dyspnea symptoms and providing tolerable safety profiles in patients with non-small cell lung cancer (NSCLC) with malignant pleural effusion. A further prospective trial is warranted to evaluate the clinical effects of intrapleural docetaxel therapy in order to compare it with other treatment modalities.
Keywords: Docetaxel; malignant pleural effusion; pleurodesis.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Lorenzo MJ, Modesto M, Perez J et al Quality‐of‐life assessment in malignant pleural effusion treated with indwelling pleural catheter: A prospective study. Palliat Med 2014; 28: 326–34. - PubMed
-
- Thomas JM, Musani AI. Malignant pleural effusions: A review. Clin Chest Med 2013; 34: 459–71. - PubMed
-
- Feller‐Kopman DJ, Reddy CB, DeCamp MM et al Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018; 198: 839–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

