New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections
- PMID: 31389664
- PMCID: PMC7159701
- DOI: 10.1002/asia.201900841
New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections
Abstract
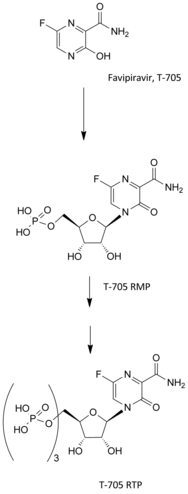
Eight different compounds, all nucleoside analogues, could presently be considered as potential drug candidates for the treatment of Ebola virus (EBOV) and/or other hemorrhagic fever virus (HFV) infections. They can be considered as either (i) adenine analogues (3-deazaneplanocin A, galidesivir, GS-6620 and remdesivir) or (ii) guanine analogues containing the carboxamide entity (ribavirin, EICAR, pyrazofurin and favipiravir). All eight owe their mechanism of action to hydrogen bonded base pairing with either (i) uracil or (ii) cytosine. Four out of the eight compounds (galidesivir, GS-6620, remdesivir and pyrazofurin) are C-nucleosides, and two of them (GS-6620, remdesivir) also contain a phosphoramidate part. The C-nucleoside and phosphoramidate (and for the adenine analogues the 1'-cyano group as well) may be considered as essential attributes for their antiviral activity.
Keywords: antivirals; ebola; hemorrhagic fever viruses; nucleoside analogues.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Andrei G., De Clercq E., Antiviral Res. 1993, 22, 45–75. - PubMed
-
- McCormick J. B., King I. J., Webb P. A., Scribner C. L., Craven R. B., Johnson K. M., Elliott L. H., Belmont-Williams R., N. Engl. J. Med. 1986, 314, 20–26. - PubMed
-
- Sidwell R. W., Huffman J. H., Khare G. P., Allen L. B., Witkowski J. T., Robins R. K., Science 1972, 177, 705–706. - PubMed
-
- De Clercq E., Med. Res. Rev. 2009, 29, 611–645. - PubMed
-
- Matsuda A., Minakawa N., Sasaki T., Ueda T., Chem. Pharm. Bull. 1988, 36, 2730–2733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical