Results and challenges of Cytochrome P450 2D6 (CYP2D6) testing in an ethnically diverse South Florida population
- PMID: 31389673
- PMCID: PMC6732280
- DOI: 10.1002/mgg3.922
Results and challenges of Cytochrome P450 2D6 (CYP2D6) testing in an ethnically diverse South Florida population
Abstract
Background: This study focuses on the implementation of CYP2D6 genetic test profiling and the challenges associated with using standard pharmacogenetics panels in a diverse South Florida population.
Methods: A total of 413 participants were recruited to participate in this study through Nicklaus Children's Hospital. Buccal swabs were collected and tested using an extended CYP2D6 panel including 22 alleles. Phenotype, genotype, and allelic frequencies were compared among different racial and ethnic groups.
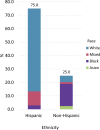
Results: The majority of participants (75.0%) self-identified as Hispanics. Four alleles, CYP2D6*4, *17, *41, and *2A, showed a statistically significant difference between White Hispanics and Black Non-Hispanics. Aggregate frequency of all alleles with decreased function varied between 2.8% and 50.0% in different racial and ethnic groups. Additionally, rare allele combinations were observed in this South Florida cohort.
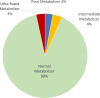
Conclusions: The heterogeneity among Hispanic groups demonstrated in previous literature and by this study reflects the complexity of ethnicity and suggests that a more granular categorization is needed, one based on ancestry and migration history rather than primary language. Overall, we have determined that there are statistically significant differences in CYP2D6 allele frequencies in the distinct racial and ethnic populations of South Florida, demonstrating a unique genetic makeup within South Florida. However, overall, the frequencies of Poor Metabolizer, Normal Metabolizer, Intermediate Metabolizer, and Ultrarapid Metabolizer did not differ between racial and ethnic groups at a statistically significant level.
Keywords: CYP2D6; Hispanic; ethnicity; intermediate metabolizers; normal metabolizers; pharmacogenetics; poor metabolizers; race; ultrarapid metabolizers.
© 2019 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
None declared.
Figures

References
-
- Barron, C. R. , Tonarelli, S. , Delozier, A. , Briones, D. F. , Su, B. B. , Rubin, L. P. , & Xu, C. (2016). Pharmacogenetics of antidepressants, A review of significant genetic variants in different populations. Clinical Depression, 2 10.4172/2572-0791.1000109 - DOI
-
- Bell, G. C. , Caudle, K. E. , Whirl‐Carrillo, M. , Gordon, R. J. , Hikino, K. , Prows, C. A. , … Schwab, M. (2017). Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clinical Pharmacology and Therapeutics, 102, 213–218. 10.1002/cpt.598 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

