Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia
- PMID: 31389735
- PMCID: PMC6879907
- DOI: 10.1152/ajplung.00080.2018
Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia
Abstract
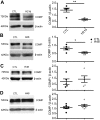
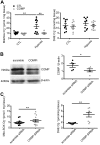
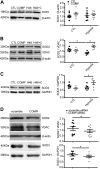
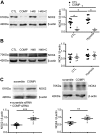
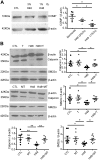
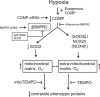
Changes in reactive oxygen species and extracellular matrix seem to participate in pulmonary hypertension development. Because we recently reported evidence for chronic hypoxia decreasing expression of cartilage oligomeric matrix protein (COMP) and evidence for this controlling loss of pulmonary arterial smooth muscle bone morphogenetic protein receptor-2 (BMPR2) and contractile phenotype proteins, we examined if changes in superoxide metabolism could be an important factor in a bovine pulmonary artery (BPA), organoid cultured under hypoxia for 48 h model. Hypoxia (3% O2) caused a depletion of COMP in BPA, but not in bovine coronary arteries. Knockdown of COMP by small-interfering RNA (siRNA) increased BPA levels of mitochondrial and extra-mitochondrial superoxide detected by MitoSOX and dihydroethidium (DHE) HPLC products. COMP siRNA-treated BPA showed reduced levels of SOD2 and SOD3 and increased levels of NADPH oxidases NOX2 and NOX4. Hypoxia increased BPA levels of MitoSOX-detected superoxide and caused changes in NOX2 and SOD2 expression similar to COMP siRNA, and exogenous COMP (0.5 μM) prevented the effects of hypoxia. In the presence of COMP, BMPR2 siRNA-treated BPA showed increases in superoxide detected by MitoSOX and depletion of SOD2. Superoxide scavengers (0.5 μM TEMPO or mitoTEMPO) maintained the expression of contractile phenotype proteins calponin and SM22α decreased by 48 h hypoxia (1% O2). Adenoviral delivery of BMPR2 to rat pulmonary artery smooth muscle cells prevented the depletion of calponin and SM22α by COMP siRNA. Thus, COMP regulation of BMPR2 appears to have an important role in controlling hypoxia-elicited changes in BPA superoxide and its potential regulation of contractile phenotype proteins.
Keywords: NADPH oxidases; extracellular matrix; pulmonary hypertension; smooth muscle phenotype; superoxide dismutase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121: 2661–2671, 2010. doi: 10.1161/CIRCULATIONAHA.109.916098. - DOI - PMC - PubMed
-
- Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010. doi: 10.1152/ajplung.00276.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

