Endotracheal tube mucus as a source of airway mucus for rheological study
- PMID: 31389736
- PMCID: PMC6842913
- DOI: 10.1152/ajplung.00238.2019
Endotracheal tube mucus as a source of airway mucus for rheological study
Abstract
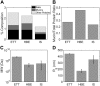
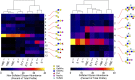
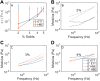
Muco-obstructive lung diseases (MOLDs), like cystic fibrosis and chronic obstructive pulmonary disease, affect a spectrum of subjects globally. In MOLDs, the airway mucus becomes hyperconcentrated, increasing osmotic and viscoelastic moduli and impairing mucus clearance. MOLD research requires relevant sources of healthy airway mucus for experimental manipulation and analysis. Mucus collected from endotracheal tubes (ETT) may represent such a source with benefits, e.g., in vivo production, over canonical sample types such as sputum or human bronchial epithelial (HBE) mucus. Ionic and biochemical compositions of ETT mucus from healthy human subjects were characterized and a stock of pooled ETT samples generated. Pooled ETT mucus exhibited concentration-dependent rheologic properties that agreed across spatial scales with reported individual ETT samples and HBE mucus. We suggest that the practical benefits compared with other sample types make ETT mucus potentially useful for MOLD research.
Keywords: cystic fibrosis; muco-obstructive lung disease; mucus; mucus biochemistry; mucus biophysics.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Comment in
-
Reliably sourced airway mucus.Am J Physiol Lung Cell Mol Physiol. 2019 Oct 1;317(4):L496-L497. doi: 10.1152/ajplung.00362.2019. Epub 2019 Sep 11. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31508979 Free PMC article. No abstract available.
References
-
- Alexis NE, Wells H, Siperly E, Goldstein B, Henderson AG, Peden DB. Baseline sputum parameters in normals, asthmatics, COPD, atopics, smokers and ex-smokers (Abstract) J Allergy Clin Immunol 137: AB208, 2016. doi: 10.1016/j.jaci.2015.12.1121. - DOI
-
- Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am J Respir Crit Care Med 192: 182–190, 2015. doi: 10.1164/rccm.201412-2230OC. - DOI - PMC - PubMed
-
- Aoki K, Porterfield M, Lee SS, Dong B, Nguyen K, McGlamry KH, Tiemeyer M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J Biol Chem 283: 30385–30400, 2008. doi: 10.1074/jbc.M804925200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

