Glutamatergic input varies with phrenic motor neuron size
- PMID: 31389739
- PMCID: PMC6843086
- DOI: 10.1152/jn.00430.2019
Glutamatergic input varies with phrenic motor neuron size
Abstract
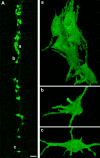
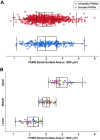
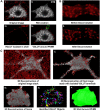
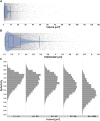
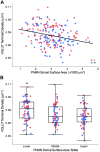
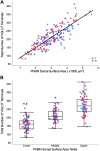
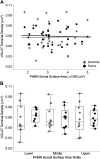
Like all skeletal muscles, the diaphragm muscle accomplishes a range of motor behaviors by recruiting different motor unit types in an orderly fashion. Recruitment of phrenic motor neurons (PhMNs) is generally assumed to be based primarily on the intrinsic properties of PhMNs with an equal distribution of descending excitatory inputs to all PhMNs. However, differences in presynaptic excitatory input across PhMNs of varying sizes could also contribute to the orderly recruitment pattern. In the spinal cord of Sprague-Dawley rats, we retrogradely labeled PhMNs using cholera toxin B (CTB) and validated a robust confocal imaging-based technique that utilizes semiautomated processing to identify presynaptic glutamatergic (Glu) terminals within a defined distance around the somal membrane of PhMNs of varying size. Our results revealed an ~10% higher density of Glu terminals at PhMNs in the lower tertile of somal surface area. These smaller PhMNs are likely recruited first to accomplish lower force ventilatory behaviors of the diaphragm as compared with larger PhMNs in the upper tertile that are recruited to accomplish higher force expulsive behaviors. These results suggest that differences in excitatory synaptic input to PhMNs may also contribute to the orderly recruitment of diaphragm motor units.NEW & NOTEWORTHY The distribution of excitatory glutamatergic synaptic input to phrenic motor neurons differs across motor neurons of varying size. These findings support the size principle of motor unit recruitment that underlies graded force generation in a muscle, which is based on intrinsic electrophysiological properties of motor neurons resulting from differences in somal surface area. A higher density of glutamatergic inputs at smaller, more excitable motor neurons substantiates the earlier and more frequent recruitment of these units.
Keywords: 3D-reconstruction; glutamate; neuromotor control; phrenic motor neurons; presynaptic inputs; spinal cord.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Bae YC, Nakamura T, Ihn HJ, Choi MH, Yoshida A, Moritani M, Honma S, Shigenaga Y. Distribution pattern of inhibitory and excitatory synapses in the dendritic tree of single masseter α-motoneurons in the cat. J Comp Neurol 414: 454–468, 1999. doi: 10.1002/(SICI)1096-9861(19991129)414:4<454:AID-CNE3>3.0.CO;2-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

