Discovery of β-Arrestin Biased, Orally Bioavailable, and CNS Penetrant Neurotensin Receptor 1 (NTR1) Allosteric Modulators
- PMID: 31390201
- PMCID: PMC7003992
- DOI: 10.1021/acs.jmedchem.9b00340
Discovery of β-Arrestin Biased, Orally Bioavailable, and CNS Penetrant Neurotensin Receptor 1 (NTR1) Allosteric Modulators
Abstract
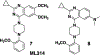
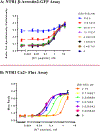
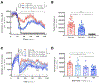
Neurotensin receptor 1 (NTR1) is a G protein coupled receptor that is widely expressed throughout the central nervous system where it acts as a neuromodulator. Neurotensin receptors have been implicated in a wide variety of CNS disorders, but despite extensive efforts to develop small molecule ligands there are few reports of such compounds. Herein we describe the optimization of a quinazoline based lead to give 18 (SBI-553), a potent and brain penetrant NTR1 allosteric modulator.
Figures



References
-
- Carraway R, Leeman SE The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem, 1973, 248, 6854–6861. - PubMed
-
- Uhl GR Distribution of neurotensin and its receptor in the central nervous system. Ann. N. Y. Acad. Sci. 1982, 400, 132–149. - PubMed
-
- Quirion R, Rowe WB, Lapchak PA, Araujo DM, and Beaudet A Distribution of neurotensin receptors in mammalian brain. What it is telling us about its interactions with other neurotransmitter systems. Ann. N. Y. Acad. Sci. 1992, 668, 109–119. - PubMed
-
- Vincent J-P; Mazella J; Kitabgi P Neurotensin and neurotensin receptors. Trends Pharmacol. Sci, 1999, 20, 302–309. - PubMed
-
- Hermans E; Maloteaux JM Mechanisms of Regulation of Neurotensin Receptors. Pharmacol. Ther, 1998, 79, 89–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

