Phase II California Cancer Consortium Trial of Gemcitabine-Eribulin Combination in Cisplatin-Ineligible Patients With Metastatic Urothelial Carcinoma: Final Report (NCI-9653)
- PMID: 31390274
- PMCID: PMC7006847
- DOI: 10.1200/JCO.19.00861
Phase II California Cancer Consortium Trial of Gemcitabine-Eribulin Combination in Cisplatin-Ineligible Patients With Metastatic Urothelial Carcinoma: Final Report (NCI-9653)
Abstract
Purpose: Patients with metastatic urothelial carcinoma are often ineligible for cisplatin-based treatments. A National Cancer Institute Cancer Therapy Evaluation Program-sponsored trial assessed the tolerability and efficacy of a gemcitabine-eribulin combination in this population.
Methods: Patients with treatment-naïve advanced or recurrent metastatic urothelial carcinoma of the bladder, ureter, or urethra not amenable to curative surgery and not candidates for cisplatin-based therapy were eligible. Cisplatin ineligibility was defined as creatinine clearance less than 60 mL/min (but ≥ 30 mL/min), grade 2 neuropathy, or grade 2 hearing loss. Treatment was gemcitabine 1,000 mg/m2 intravenously followed by eribulin 1.4 mg/m2, both on days 1 and 8, repeated in 21-day cycles until progression or unacceptable toxicity. A Simon two-stage phase II trial design was used to distinguish between Response Evaluation Criteria in Solid Tumors, version 1.1 objective response rates of 20% versus 50%.
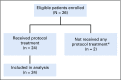
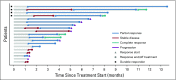
Results: Between June 2015 and March 2017, 24 eligible patients with a median age of 73 years (range, 62 to 88 years) underwent therapy. Performance status of 0, 1, or 2 was seen in 11, 11, and two patients, respectively. Sites of disease included: lymph nodes, 16; lungs, nine; liver, seven; bladder, five; bones, two. Median number of cycles received was four (range, one to 16). Of 24 patients, 12 were confirmed responders; the observed objective response rate was 50% (95% CI, 29% to 71%). Median overall survival was 11.9 months (95% CI, 5.6 to 20.4 months), and median progression-free survival was 5.3 months (95% CI, 4.5 to 6.7 months). The most common treatment-related any-grade toxicities were fatigue (83% of patients), neutropenia (79%), anemia (63%), alopecia (50%), elevated AST (50%), and constipation, nausea, and thrombocytopenia (42% each).
Conclusion: Gemcitabine-eribulin treatment response and survival for cisplatin-ineligible patients compare favorably to other regimens. Additional research is needed.
Trial registration: ClinicalTrials.gov NCT02178241.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Boyle P, Levin B (eds): World Cancer Report 2008. Lyon, France, IARC Press, 2008.
-
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. - PubMed
-
- Bohnsack O, Hoos A, Ludajic K: Adaptation and modification of the immune related response criteria (IRRC): IrRECIST. J Clin Oncol 32, 2014 (suppl 15; abstr e22121)
-
- Sternberg CN. Muscle invasive and metastatic bladder cancer. Ann Oncol. 2006;17(suppl 10):x23–x30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

