Evaluation of flow cytometry for the detection of bacteria in biological fluids
- PMID: 31390352
- PMCID: PMC6685611
- DOI: 10.1371/journal.pone.0220307
Evaluation of flow cytometry for the detection of bacteria in biological fluids
Abstract
Objectives: Conventional microbiological procedures for the isolation of bacteria from biological fluids consist of culture on solid media and enrichment broth. However, these methods can delay the microbiological identification for up to 4 days. The aim of this study was to evaluate the analytical performance of Sysmex UF500i (Sysmex, Kobe, Japan) as a screening method for the detection of bacteria in different biological fluids in comparison with direct Gram staining and the conventional culture on solid media and enrichment broth.
Methods: A total of 479 biological fluid samples were included in the study (180 ascitic, 131 amniotic, 56 synovial, 40 cerebrospinal, 36 pleural, 24 peritoneal, 9 bile and 3 pericardial fluids). All samples were processed by conventional culture methods and analyzed by flow cytometry. Direct Gram staining was performed in 339 samples. The amount of growth on culture was recorded for positive samples.
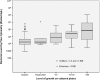
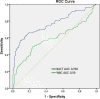
Results: Bacterial and white blood cell count by flow cytometry was significantly higher among culture positive samples and samples with a positive direct Gram stain compared to culture negative samples. Bacterial count directly correlated with the amount of growth on culture (Kruskall-Wallis H χ2(3) = 11.577, p = 0.009). The best specificity (95%) for bacterial count to predict culture positivity was achieved applying a cut-off value of 240 bacteria/μL.
Conclusions: Bacterial and white blood cell counts obtained with flow cytometry correlate with culture results in biological fluids. Bacterial count can be used as a complementary method along with the direct Gram stain to promptly detect positive samples and perform other diagnostic techniques in order to accelerate the bacterial detection and identification.
Conflict of interest statement
Sysmex España S.L. financially supported the study providing reagents and equipment. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. Elsevier; 1993;169: 839–851. 10.1016/0002-9378(93)90014-a - DOI - PubMed

