Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages
- PMID: 31390749
- PMCID: PMC6720685
- DOI: 10.3390/antiox8080281
Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages
Abstract
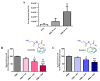
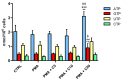
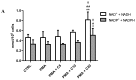
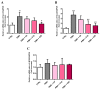
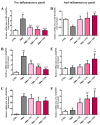
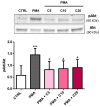
Carnosine is an endogenous dipeptide composed of β-alanine and L-histidine. This naturally occurring molecule is present at high concentrations in several mammalian excitable tissues such as muscles and brain, while it can be found at low concentrations in a few invertebrates. Carnosine has been shown to be involved in different cellular defense mechanisms including the inhibition of protein cross-linking, reactive oxygen and nitrogen species detoxification as well as the counteraction of inflammation. As a part of the immune response, macrophages are the primary cell type that is activated. These cells play a crucial role in many diseases associated with oxidative stress and inflammation, including atherosclerosis, diabetes, and neurodegenerative diseases. In the present study, carnosine was first tested for its ability to counteract oxidative stress. In our experimental model, represented by RAW 264.7 macrophages challenged with phorbol 12-myristate 13-acetate (PMA) and superoxide dismutase (SOD) inhibitors, carnosine was able to decrease the intracellular concentration of superoxide anions (O2-•) as well as the expression of Nox1 and Nox2 enzyme genes. This carnosine antioxidant activity was accompanied by the attenuation of the PMA-induced Akt phosphorylation, the down-regulation of TNF-α and IL-6 mRNAs, and the up-regulation of the expression of the anti-inflammatory mediators IL-4, IL-10, and TGF-β1. Additionally, when carnosine was used at the highest dose (20 mM), there was a generalized amelioration of the macrophage energy state, evaluated through the increase both in the total nucleoside triphosphate concentrations and the sum of the pool of intracellular nicotinic coenzymes. Finally, carnosine was able to decrease the oxidized (NADP+)/reduced (NADPH) ratio of nicotinamide adenine dinucleotide phosphate in a concentration dependent manner, indicating a strong inhibitory effect of this molecule towards the main source of reactive oxygen species in macrophages. Our data suggest a multimodal mechanism of action of carnosine underlying its beneficial effects on macrophage cells under oxidative stress and inflammation conditions.
Keywords: antioxidants; carnosine; inflammation; macrophages; oxidative stress; superoxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- De Campos R.P., Siegel J.M., Fresta C.G., Caruso G., Da Silva J.A., Lunte S.M. Indirect detection of superoxide in raw 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015;407:7003–7012. doi: 10.1007/s00216-015-8865-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

