Yinang formulation versus placebo granules as a treatment for chronic kidney disease stages III-IV in patients with autosomal dominant polycystic kidney disease: study protocol for a double-blind placebo-controlled randomized clinical trial
- PMID: 31391092
- PMCID: PMC6686499
- DOI: 10.1186/s13063-019-3563-5
Yinang formulation versus placebo granules as a treatment for chronic kidney disease stages III-IV in patients with autosomal dominant polycystic kidney disease: study protocol for a double-blind placebo-controlled randomized clinical trial
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common potentially life-threatening inherited kidney diseases. It is the fourth most common cause of end-stage renal disease requiring renal replacement therapy. There are few management options for controlling disease progression. Hence, identification of alternative treatments for patients is important. The Chinese herbal yinang formulation (YNF), which is derived from a Chinese patent medicine, appears to have a satisfactory effect in treating ADPKD. Because a considerable proportion of ADPKD patients presenting with chronic kidney disease (CKD) stages III-IV are diagnosed with the spleen, kidney deficiency, and blood stasis syndrome according to the diagnostic criteria of traditional Chinese medicine (TCM), we hypothesize that YNF may be a complementary drug for ADPKD patients with the corresponding syndrome. Therefore, we have designed a strict clinical trial to evaluate the safety and efficacy of YNF for ADPKD patients with CKD stages III-IV exhibiting the TCM syndrome of spleen, kidney deficiency, and blood stasis.
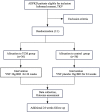
Methods/design: This is a multi-center prospective double-blind randomized controlled trial. The total target sample size is planned to be 72 participants, with a balanced treatment allocation (1:1). The experimental intervention will be YNF plus conventional therapy and the control intervention will be a placebo plus conventional therapy for 24 weeks. An additional 24 weeks of follow-up will be conducted after treatment completion. The primary outcome will be the estimated glomerular filtration rate (eGFR). Changes in total kidney volume (TKV), serum creatinine (Scr), blood urea nitrogen (BUN), TCM symptoms, and pain will be the secondary outcomes. Adverse events (AEs) will be monitored throughout the trial.
Discussion: This study will be the first placebo-controlled randomized controlled trial to assess whether YNF plus conventional therapy has a beneficial effect on eGFR, TKV, Scr, and BUN, and whether it can alleviate TCM clinical symptoms, reduce ADPKD-related pain, and reduce the frequency of AEs for ADPKD patients with CKD stages III-IV with the spleen, kidney deficiency, and blood stasis syndrome. The results of this trial may provide an evidence-based recommendation for clinicians.
Trial registration: Chinese Clinical Trials Register, ChiCTR-INR-16009914 . Registered on 18 November 2016.
Keywords: Autosomal dominant polycystic kidney disease; Efficacy; Randomized controlled trial; Safety; Stages III–IV chronic kidney disease; Yinang formulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Meijer E, Jong PE, Peters DJ, Gansevoort RT. Better understanding of ADPKD results in potential new treatment options:ready for the cure. J Nephrol. 2008;21(2):133–138. - PubMed
-
- Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a kidney Disease: improving global outcomes (KDIGO) controversies conference. Kid Int. 2015;88(1):17–27. doi: 10.1038/ki.2015.59. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous