Prohibitin is a prognostic marker and therapeutic target to block chemotherapy resistance in Wilms' tumor
- PMID: 31391345
- PMCID: PMC6693841
- DOI: 10.1172/jci.insight.127098
Prohibitin is a prognostic marker and therapeutic target to block chemotherapy resistance in Wilms' tumor
Abstract
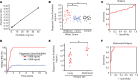
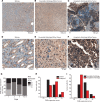
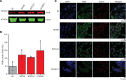
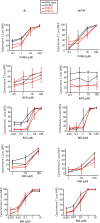
Wilms' tumor is the most common type of childhood kidney cancer. To improve risk stratification and identify novel therapeutic targets for patients with Wilms' tumor, we used high-resolution mass spectrometry proteomics to identify urine tumor markers associated with Wilms' tumor relapse. We determined the urine proteomes at diagnosis of 49 patients with Wilms' tumor, non-Wilms' tumor renal tumors, and age-matched controls, leading to the quantitation of 6520 urine proteins. Supervised analysis revealed specific urine markers of renal rhabdoid tumors, kidney clear cell sarcomas, renal cell carcinomas as well as those detected in patients with cured and relapsed Wilms' tumor. In particular, urine prohibitin was significantly elevated at diagnosis in patients with relapsed as compared with cured Wilms' tumor. In a validation cohort of 139 patients, a specific urine prohibitin ELISA demonstrated that prohibitin concentrations greater than 998 ng/mL at diagnosis were significantly associated with ultimate Wilms' tumor relapse. Immunohistochemical analysis revealed that prohibitin was highly expressed in primary Wilms' tumor specimens and associated with disease stage. Using functional genetic experiments, we found that prohibitin was required for the growth and survival of Wilms' tumor cells. Overexpression of prohibitin was sufficient to block intrinsic mitochondrial apoptosis and to cause resistance to diverse chemotherapy drugs, at least in part by dysregulating factors that control apoptotic cytochrome c release from mitochondrial cristae. Thus, urine prohibitin may improve therapy stratification, noninvasive monitoring of treatment response, and early disease detection. In addition, therapeutic targeting of chemotherapy resistance induced by prohibitin dysregulation may offer improved therapies for patients with Wilms' and other relapsed or refractory tumors.
Keywords: Apoptosis; Cancer; Cell Biology; Oncology.
Conflict of interest statement
Figures







References
-
- Grundy PE, Telzerow PE, Breslow N, Moksness J, Huff V, Paterson MC. Loss of heterozygosity for chromosomes 16q and 1p in Wilms’ tumors predicts an adverse outcome. Cancer Res. 1994;54(9):2331–2333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

