High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism
- PMID: 31391499
- PMCID: PMC6686006
- DOI: 10.1038/s41598-019-47836-8
High Glucose Environments Interfere with Bone Marrow-Derived Macrophage Inflammatory Mediator Release, the TLR4 Pathway and Glucose Metabolism
Abstract
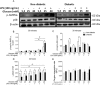
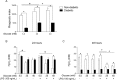
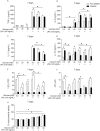
Macrophages may be a crucial aspect of diabetic complications associated with the inflammatory response. In this study, we examined how hyperglycaemia, a common aspect of diabetes, modulates bone marrow-derived macrophages (BMDMs) under an inflammatory stimulus. To perform this study, BMDMs from non-diabetic and diabetic (60 mg/kg alloxan, i.v.) male C57BL/6 mice (CEUA/FCF/USP-488) were cultured under normal (5.5 mM) and high glucose (HG, 25 or 40 mM) conditions and stimulated or not stimulated with lipopolysaccharide (LPS, 100 ng/mL). Compared to the BMDMs from the normoglycaemic mice, the LPS-stimulated BMDMs from the diabetic mice presented reduced TLR4 expression on the cell surface, lower phagocytic capacity, and reduced secretion of NO and lactate but greater oxygen consumption and greater phosphorylation of p46 SAPK/JNK, p42 ERK MAPK, pAKT and pPKC-δ. When the BMDMs from the non-diabetic mice were cultured under high-glucose conditions and stimulated with LPS, TLR4 expression was reduced on the cell surface and NO and H2O2 levels were reduced. In contrast, the diabetic BMDMs cultured under high glucose conditions presented increased levels of lactate and reduced phosphorylation of AKT, PKC-δ and p46 SAPK/JNK but enhanced phosphorylation of the p46 subunit of SAPK/JNK after LPS stimulation. High glucose levels appear to modify macrophage behaviour, affecting different aspects of diabetic and healthy BMDMs under the same LPS stimulus. Thus, hyperglycaemia leaves a glucose legacy, altering the basal steady state of macrophages.
Conflict of interest statement
The authors declare no competing interests.
Figures

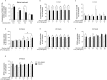



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

