Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model
- PMID: 31391536
- PMCID: PMC6685943
- DOI: 10.1038/s41598-019-47389-w
Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model
Abstract
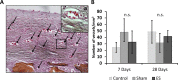
Different species respond differently to severe injury, such as limb loss. In species that regenerate, limb loss is met with complete restoration of the limbs' form and function, whereas in mammals the amputated limb's stump heals and scars. In in vitro studies, electrical stimulation (EStim) has been shown to promote cell migration, and osteo- and chondrogenesis. In in vivo studies, after limb amputation, EStim causes significant new bone, cartilage and vessel growth. Here, in a rat model, the stumps of amputated rat limbs were exposed to EStim, and we measured extracellular matrix (ECM) deposition, macrophage distribution, cell proliferation and gene expression changes at early (3 and 7 days) and later stages (28 days). We found that EStim caused differences in ECM deposition, with less condensed collagen fibrils, and modified macrophage response by changing M1 to M2 macrophage ratio. The number of proliferating cells was increased in EStim treated stumps 7 days after amputation, and transcriptome data strongly supported our histological findings, with activated gene pathways known to play key roles in embryonic development and regeneration. In conclusion, our findings support the hypothesis that EStim shifts injury response from healing/scarring towards regeneration. A better understanding of if and how EStim controls these changes, could lead to strategies that replace scarring with regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carlson, B. M. Principles of regenerative biology (Elsevier/Academic, Amsterdam, London, 2007).
-
- Carlson BM. Stimulation of regeneration in mammals: pipe dream or realistic goal? Wound repair and regeneration: official publication of the Wound Healing. Society [and] the European Tissue Repair Society. 1998;6:425–433. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

