Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review
- PMID: 31392056
- PMCID: PMC6657309
- DOI: 10.21037/jgo.2019.03.10
Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review
Abstract
Background: The recognition of distinct molecular subgroups within cholangiocarcinoma (CC), along with the increasing availability of targeted therapies, suggests that further characterization of the prevalence and prognosis of frequently occurring subgroups may assist with the development of more effective treatment approaches for the management of CC. A systematic review was performed to investigate the prevalence of isocitrate dehydrogenase 1 (IDH1) mutations (mIDH1) in patients with CC, the possible clinical and prognostic significance of mIDH1, and the presence of co-mutations in tumors with mIDH1.
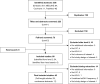
Methods: This review was conducted using the Cochrane dual-reviewer methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol (PRISMA-P) guidelines. Searches were performed in Embase, MEDLINE, the Cochrane Central Trials Register and Database of Systematic Reviews, and other Cochrane Library assets using terms for CC and mIDH1 with no language or date restrictions for articles published up to December 31, 2017. Searches were also performed of abstracts presented at the following conferences in 2016 and 2017: American Society of Clinical Oncology (ASCO), ASCO-Gastrointestinal Cancers Symposium (ASCO-GI), the European Society for Medical Oncology (ESMO), and ESMO-Asia. Screening was performed separately by two reviewers and cross-checked. Any discrepancies between reviewers were resolved by a senior researcher. Data from all selected references were recorded in a data extraction grid.
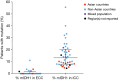
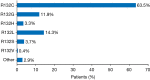
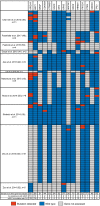
Results: A total of 46 publications met the inclusion criteria and were included in the systematic review. Of these publications, 45 reported the frequency of mIDH1 among a total sample of 5,393 patients with CC. mIDH1 was enriched in intrahepatic CC (ICC), with 552 (13.1%; 95% CI, 12.1-14.2) of the 4,214 patients with ICC having the mutation compared with 9 (0.8%; 95% CI, 0.4-1.5%) of the 1,123 patients with extrahepatic CC (ECC). The percentage of females with mIDH1 CC (66.2%; 95% CI, 57.7-73.7%) was higher than in the overall CC population (44.4%). The frequency of mIDH1 in patients with ICC reported in individual studies ranged from 4.5-55.6%, and a significantly higher frequency was reported in non-Asian centers compared with Asian centers (weighted mean, 16.5% vs. 8.8%; P<0.001). The prevalence of mIDH1 in patients with ICC at USA centers was 18.0% (95% CI, 16.4-19.8%). Eleven publications reported the prevalence of co-mutations in patients with mIDH1 ICC, with the most frequent being AT-rich interactive domain-containing protein 1A (ARID1A) (22.0%), BRCA1-associated protein 1 (BAP1) (15.5%), and PBRM1 (13.3%). Eight publications investigated the possible prognostic significance of mIDH1. None of the studies reported a statistically significant association between mIDH1 and overall survival (OS), progression-free survival (PFS), or time to progression.
Conclusions: This systematic review substantiates the prevalence of mIDH1 in CC and further characterizes clinical, pathologic, and genetic covariates within this sub-population. Co-mutation data may inform future studies of mechanisms of response and resistance to mIDH1-targeted therapies.
Keywords: Cholangiocarcinoma (CC); clinical prognosis; isocitrate dehydrogenase 1 (IDH1); prevalence; systematic review.
Conflict of interest statement
Conflicts of Interest: AN Boscoe is an employee and shareholder of Agios Pharmaceuticals Inc. C Rolland is an employee of Envision Pharma Group, paid consultants to Agios Pharmaceuticals Inc in connection with this study. RK Kelley receives support (to institution) for conduct of clinical trials from: Agios, Astra Zeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Exelixis, MedImmune, Merck, QED, Novartis, Taiho; she also receives consulting fees (to individual) for advisory board/IDMC membership from Genentech/Roche and Target Pharma Solutions.
Figures
References
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
