Functional analysis of GALT variants found in classic galactosemia patients using a novel cell-free translation method
- PMID: 31392114
- PMCID: PMC6606980
- DOI: 10.1002/jmd2.12037
Functional analysis of GALT variants found in classic galactosemia patients using a novel cell-free translation method
Abstract
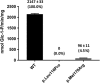
Classic galactosemia is an autosomal recessive disorder caused by deleterious variants in the galactose-1-phosphate uridylyltransferase (GALT) gene. GALT enzyme deficiency leads to an increase in the levels of galactose and its metabolites in the blood causing neurodevelopmental and other clinical complications in affected individuals. Two GALT variants NM_000155.3:c.347T>C (p.Leu116Pro) and NM_000155.3:c.533T>G (p.Met178Arg) were previously detected in Filipino patients. Here, we determine their functional effects on the GALT enzyme through in silico analysis and a novel experimental approach using a HeLa-based cell-free protein expression system. Enzyme activity was not detected for the p.Leu116Pro protein variant, while only 4.5% of wild-type activity was detected for the p.Met178Arg protein variant. Computational analysis of the variants revealed destabilizing structural effects and suggested protein misfolding as the potential mechanism of enzymological impairment. Biochemical and computational data support the classification of p.Leu116Pro and p.Met178Arg variants as pathogenic. Moreover, the protein expression method developed has utility for future studies of GALT variants.
Keywords: GALT deficiency; HeLa‐based cell‐free expression system; classic galactosemia; galactose‐1‐phosphate uridylyltransferase; missense variants.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Classic Galactosemia: Clinical and Computational Characterization of a Novel GALT Missense Variant (p.A303D) and a Literature Review.Int J Mol Sci. 2023 Dec 12;24(24):17388. doi: 10.3390/ijms242417388. Int J Mol Sci. 2023. PMID: 38139222 Free PMC article. Review.
-
Novel GALT variations and genetic spectrum in Turkish population with the correlation of genotype and phenotype.Ann Hum Genet. 2023 Nov;87(6):285-294. doi: 10.1111/ahg.12523. Epub 2023 Aug 11. Ann Hum Genet. 2023. PMID: 37563963
-
Galactosemia: when is it a newborn screening emergency?Mol Genet Metab. 2012 May;106(1):7-11. doi: 10.1016/j.ymgme.2012.03.007. Epub 2012 Mar 21. Mol Genet Metab. 2012. PMID: 22483615 Review.
-
An extensive computational approach to analyze and characterize the functional mutations in the galactose-1-phosphate uridyl transferase (GALT) protein responsible for classical galactosemia.Comput Biol Med. 2020 Feb;117:103583. doi: 10.1016/j.compbiomed.2019.103583. Epub 2019 Dec 13. Comput Biol Med. 2020. PMID: 32072977
-
Functional and structural impact of the most prevalent missense mutations in classic galactosemia.Mol Genet Genomic Med. 2014 Nov;2(6):484-96. doi: 10.1002/mgg3.94. Epub 2014 Jun 23. Mol Genet Genomic Med. 2014. PMID: 25614870 Free PMC article.
Cited by
-
Clinical and biochemical phenotypes, genotypes, and long-term outcomes of individuals with galactosemia type I from a single metabolic genetics center in Alberta.Mol Genet Metab Rep. 2024 Jan 25;38:101055. doi: 10.1016/j.ymgmr.2024.101055. eCollection 2024 Mar. Mol Genet Metab Rep. 2024. PMID: 38469090 Free PMC article.
-
A case report of classic galactosemia with a GALT gene variant and a literature review.BMC Pediatr. 2024 May 22;24(1):352. doi: 10.1186/s12887-024-04769-0. BMC Pediatr. 2024. PMID: 38778342 Free PMC article. Review.
References
-
- Tyfield L, Reichardt J, Fridovich‐Keil J, et al. Classical galactosemia and mutations at the galactose‐1‐phosphate uridyl transferase (GALT) gene. Hum Mutat. 1999;13:417‐430. - PubMed
-
- Berry GT. Classic galactosemia and clinical variant galactosemia In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle, WA: University of Washington, Seattle; 2017:1993‐2018. - PubMed
-
- Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885‐43888. - PubMed
-
- McCorvie TJ, Timson DJ. The structural and molecular biology of type I galactosemia: enzymology of galactose 1‐phosphate uridylyltransferase. IUBMB Life. 2011;63:694‐700. - PubMed
LinkOut - more resources
Full Text Sources

