Identification of cysteine thiol-based linkages in ADAMTS13 in support of a non-proteolytic regulation of von Willebrand factor
- PMID: 31393047
- PMCID: PMC6916347
- DOI: 10.1111/jth.14602
Identification of cysteine thiol-based linkages in ADAMTS13 in support of a non-proteolytic regulation of von Willebrand factor
Abstract
Background: ADAMTS13, a plasma metalloprotease, cleaves von Willebrand factor (VWF) to regulate its function. Additionally, ADAMTS13 is thought to regulate lateral association of VWF multimers to form fibrillar structures through its free thiols.
Objective: The purpose of the present study is to obtain direct evidence for ADAMTS13 to engage in thiol/disulfide exchange reactions.
Methods: Covalent complexes between ADAMTS13 and VWF were determined by agarose gel electrophoresis under nonreducing conditions. Free thiols in ADAMST13 were identified by a reversed phase high-performance liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry system.
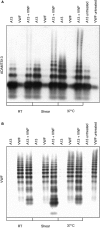
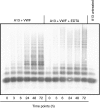
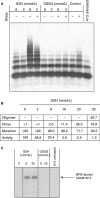
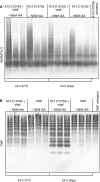
Results: We demonstrate formation of covalent linkage between ADAMTS13 and VWF, which is time, concentration, temperature, and shear dependent. This interaction is independent of proteolytic activity of ADAMTS13 but depends on the C-terminal domains comprising the fifth through eighth thrombospondin type 1 repeats and C1r/C1s, Uegf, Bmp1 (CUB) domains. The interaction can be blocked by thiol-reactive agents, indicating that association is accomplished through disulfide bridge formation. Several partially reduced free thiols are identified in ADAMTS13, with cysteines 1254 and 1275 being the most prominent, although a point mutation (C1275S) in ADAMTS13 does not alter its ability to form covalent linkages with VWF. This suggests functionally relevant disulfide plasticity in ADAMTS13. Interestingly, ADAMTS13 also forms homo-oligomers under the same conditions as required for the generation of hetero-oligomeric complexes of ADAMTS13 and VWF.
Conclusions: Our results suggest that a dynamic network of free thiols in ADAMTS13 undergoing intra- and inter-molecular redox reactions may add another layer of regulation to VWF function under various conditions.
Keywords: ADAMTS13; catalysis-independent regulation; free thiols; homo-oligomerization; von Willebrand factor.
© 2019 Shire International GmbH. Journal of Thrombosis and Haemostasis published by Wiley Periodicals, Inc. on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Hanspeter Rottensteiner, Brigit K. Seyfried, Stefan Kaufmann, Christian Fiedler, Barbara Plaimauer, and Friedrich Scheiflinger are employees of Baxalta Innovations GmbH, a member of the Takeda group of companies, Vienna, Austria. X. Long Zheng is a member of the speaker bureau of Alexion, serves as a consultant for Ablynx/Sanofi, and is the founder of Clotsolution, Inc. Jing‐Fei Dong has nothing to disclose.
Figures


References
-
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–2114. - PubMed
-
- Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

