Efficacy of denosumab for restoring normal bone mineral density in women receiving adjuvant aromatase inhibitors for early breast cancer
- PMID: 31393399
- PMCID: PMC6708609
- DOI: 10.1097/MD.0000000000016770
Efficacy of denosumab for restoring normal bone mineral density in women receiving adjuvant aromatase inhibitors for early breast cancer
Abstract
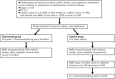
Background: Osteoporosis is a major side effect of aromatase inhibitors (AIs), which are greatly effective in the treatment of breast cancer. However, there are no satisfactory measures against osteoporosis. In this multicenter, randomized, comparative study, we evaluate the efficacy of denosumab for preventing loss of bone mineral density (BMD) induced by adjuvant therapy with AI s in breast cancer patients with normal BMD.
Patients and methods: The bone loss-suppressing effect of denosumab will be comparatively evaluated in postmenopausal patients scheduled to receive letrozole or anastrozole as a postoperative endocrine therapy for stage I-IIIA hormone-sensitive breast cancer and a control group. Patients will be administered letrozole 2.5 mg or anastrozole 1 mg once a day, and the treatment will be continued for 5 years unless recurrence, secondary cancer, or unacceptable toxicity develops. Patients in the denosumab group will receive a subcutaneous injection of 60 mg of denosumab every 6 months. The primary endpoint is the rate of change in the lumbar spine (L1-L4) BMD, as determined by dual-energy X-ray absorptiometry (DXA), 12 months after the start of the injection. The secondary endpoints were ETHICS AND DISSEMINATION:: The protocol was approved by the institutional review boards of Kyoto Prefectural University of Medicine and all the participating faculties. Written informed consent was obtained from all patients before registration, in accordance with the Declaration of Helsinki. Results of the study will be disseminated via publications in peer-reviewed journals.
Trial registration: Clinical Trials.gov Identifier: NCT03324932, Japan Registry of Clinical Trial (jRCT): CRB5180001.
Conflict of interest statement
This study was conducted as the 2016–2017 acupuncture study of the Japan Breast Cancer Society. There is no conflict of interest to be disclosed between this study and the Japan Breast Cancer Society. TT received Research grant from Chugai, Taiho and Daiichi Sankyo. ST received research grant from Daiichi Sankyo. IY has received a speaker fee from Chugai and JT. NN has received research funding to Tokai University from Novartis, Bristol-Myers Squibb, Chugai, Nihon Medi-Physics, MSD, Daiichi-Sankyo, honoraria, consultancy and speaker fees from AstraZeneca, Novartis, Eisai, and Pfizer, outside the submitted work. Other authors declare that they have no conflict of interest.
Figures
References
-
- Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: Results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845–53. - PubMed
-
- Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71. - PubMed
-
- Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 2002;23:279–302. - PubMed
-
- Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

