DOLAMA study: Effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients
- PMID: 31393412
- PMCID: PMC6708975
- DOI: 10.1097/MD.0000000000016813
DOLAMA study: Effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients
Abstract
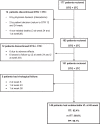
Dolutegravir (DTG) has shown effectiveness in combination with rilpivirine in with experience of antiretroviral therapy (ART) and with 3TC in naïve patients (GEMINI trial). The main objectives of this real-life study were to analyze the effectiveness and safety of 3TC plus DTG in virologically suppressed HIV-1 patients and to conduct a pharmacoeconomic analysis.We conducted an observational, retrospective and multicenter study of HIV+ patients pretreated for at least 6 months with ART that was then simplified to 3TC + DTG for any reason. We gathered data on viral loads (VLs) during exposure to the DT, calculating the rate with VL < 50 copies/mL at week 48, and on associated adverse effects.The 177 HIV+ patients were collected, 77.4% male, with average age of 48.5 years and mean count of 252.2cell/μL CD4+ nadir lymphocytes; 96.6% had VL < 50 copies/mL and 674 cells/μL CD4+ lymphocytes. Median time since HIV diagnosis was 15 years, and median ART duration was 13 years, and 34.5% of patients were on mono- or dual-therapy before the switch. At week 48, 82.4% of patients had VL < 50 cop/μL using an intention-to-treat (ITT) analysis, 89.6% according to mITT, and 96.7% according to Per-Protocol analysis. 3.3% patients had virological failure (VF). These effectiveness data and costs were compared with those for 2 reference triple therapies (DTG/ABC/3TC and EVG/cobi/FTC/TAF) in a cost minimization analysis, showing cost savings with administration of DTG+3TC (2741 &OV0556;/year vs DTG/ABC/3TC and 4164 &OV0556;/year vs EVG/cobi/FTC/TAF) and in a cost-effectiveness analysis, finding the DT to be the most cost-effective approach (ICER = -548 vs DTG/ABC/3TC and ICER = -4,627&OV0556; vs EVG/cobi/FTC/TAF)The combination of 3TC with DTG appears to be a safe and effective option for the simplification of ART in pretreated and virologically stable HIV-positive patients, being cost-effective and offering the same effectiveness as the triple therapy it replaces.
Figures
References
-
- Documento de consenso de GESIDA/plan nacional sobre el SIDA respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana. gesida-seim.org. Update January 2019.
-
- Pasquau J, Hidalgo-Tenorio C. Nuke-sparing regimens for the long-term care of HIV infection. AIDS Rev 2015;17:220–30. - PubMed
-
- Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015;15:785–92. - PubMed
-
- Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015;15:775–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials