Assessment of Long-term Distant Recurrence-Free Survival Associated With Tamoxifen Therapy in Postmenopausal Patients With Luminal A or Luminal B Breast Cancer
- PMID: 31393518
- PMCID: PMC6692699
- DOI: 10.1001/jamaoncol.2019.1856
Assessment of Long-term Distant Recurrence-Free Survival Associated With Tamoxifen Therapy in Postmenopausal Patients With Luminal A or Luminal B Breast Cancer
Abstract
Importance: Patients with estrogen receptor (ER)-positive breast cancer have a long-term risk for fatal disease. However, the tumor biological factors that influence the long-term risk and the benefit associated with endocrine therapy are not well understood.
Objective: To compare the long-term survival from tamoxifen therapy for patients with luminal A or luminal B tumor subtype.
Design, setting, and participants: Secondary analysis of patients from the Stockholm Tamoxifen (STO-3) trial conducted from 1976 to 1990, which randomized postmenopausal patients with lymph node-negative breast cancer to receive adjuvant tamoxifen or no endocrine therapy. Tumor tissue sections were assessed in 2014 using immunohistochemistry and Agilent microarrays. Only patients with luminal A or B subtype tumors were evaluated. Complete long-term follow-up data up to the end of the STO-3 trial on December 31, 2012, were obtained from the Swedish National registers. Data analysis for the secondary analysis was conducted in 2017 and 2018.
Interventions: Patients were randomized to receive at least 2 years of tamoxifen therapy or no endocrine therapy; patients without recurrence who reconsented were further randomized to 3 additional years of tamoxifen therapy or no endocrine therapy.
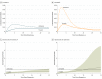
Main outcomes and measures: Distant recurrence-free interval (DRFI) by luminal A and luminal B subtype and trial arm was assessed by Kaplan-Meier analyses and time-dependent flexible parametric models to estimate time-varying hazard ratios (HRs) that were adjusted for patient and tumor characteristics.
Results: In the STO-3 treated trial arm, 183 patients had luminal A tumors and 64 patients had luminal B tumors. In the untreated arm, 153 patients had luminal A tumors and 62 had luminal B tumors. Age at diagnosis ranged from 45 to 73 years. A statistically significant difference in DRFI by trial arm was observed (log rank, P < .001 [luminal A subtype, n = 336], P = .04 [luminal B subtype, n = 126]): the 25-year DRFI for luminal A vs luminal B subtypes was 87% (95% CI, 82%-93%) vs 67% (95% CI, 56%-82%) for treated patients, and 70% (95% CI, 62%-79%) vs 54% (95% CI, 42%-70%) for untreated patients, respectively. Patients with luminal A tumors significantly benefited from tamoxifen therapy for 15 years after diagnosis (HR, 0.57; 95% CI, 0.35-0.94), and those with luminal B tumors benefited from tamoxifen therapy for 5 years (HR, 0.38; 95% CI, 0.24-0.59).
Conclusions and relevance: Patients with luminal A subtype tumors had a long-term risk of distant metastatic disease, which was reduced by tamoxifen treatment, whereas patients with luminal B tumors had an early risk of distant metastatic disease, and tamoxifen benefit attenuated over time.
Conflict of interest statement
Figures


Comment in
-
Late Recurrences After Estrogen Receptor-Positive Breast Cancer.JAMA Oncol. 2020 Feb 1;6(2):301-302. doi: 10.1001/jamaoncol.2019.5579. JAMA Oncol. 2020. PMID: 31830232 No abstract available.

