Exogenous PP2A inhibitor exacerbates the progression of nonalcoholic fatty liver disease via NOX2-dependent activation of miR21
- PMID: 31393787
- PMCID: PMC6842990
- DOI: 10.1152/ajpgi.00061.2019
Exogenous PP2A inhibitor exacerbates the progression of nonalcoholic fatty liver disease via NOX2-dependent activation of miR21
Abstract
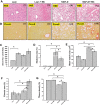
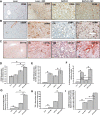
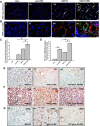
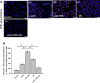
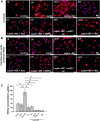
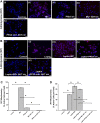
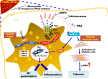
Nonalcoholic fatty liver disease (NAFLD) is an emerging global pandemic. Though significant progress has been made in unraveling the pathophysiology of the disease, the role of protein phosphatase 2A (PP2A) and its subsequent inhibition by environmental and genetic factors in NAFLD pathophysiology remains unclear. The present report tests the hypothesis that an exogenous PP2A inhibitor leads to hepatic inflammation and fibrogenesis via an NADPH oxidase 2 (NOX2)-dependent pathway in NAFLD. Results showed that microcystin (MC) administration, a potent PP2A inhibitor found in environmental exposure, led to an exacerbation of NAFLD pathology with increased CD68 immunoreactivity, the release of proinflammatory cytokines, and stellate cell activation, a process that was attenuated in mice that lacked the p47phox gene and miR21 knockout mice. Mechanistically, leptin-primed immortalized Kupffer cells (a mimicked model for an NAFLD condition) treated with apocynin or nitrone spin trap 5,5 dimethyl-1- pyrroline N-oxide (DMPO) had significantly decreased CD68 and decreased miR21 and α-smooth muscle actin levels, suggesting the role of NOX2-dependent reactive oxygen species in miR21-induced Kupffer cell activation and stellate cell pathology. Furthermore, NOX2-dependent peroxynitrite generation was primarily responsible for cellular events observed following MC exposure since incubation with phenylboronic acid attenuated miR21 levels, Kupffer cell activation, and inflammatory cytokine release. Furthermore, blocking of the AKT pathway attenuated PP2A inhibitor-induced NOX2 activation and miR21 upregulation. Taken together, we show that PP2A may have protective roles, and its inhibition exacerbates NAFLD pathology via activating NOX2-dependent peroxynitrite generation, thus increasing miR21-induced pathology.NEW & NOTEWORTHY Protein phosphatase 2A inhibition causes nonalcoholic steatohepatitis (NASH) progression via NADPH oxidase 2. In addition to a novel emchanism of action, we describe a new tool to describe NASH histopathology.
Keywords: NADPH; NAFLD; NOX-2; PP2A inhibitor; leptin; miR21; microcystin; oxidative stress; siRNA.
Conflict of interest statement
Y. Jule is a paid employee of Biocellvia, France, who designed the NASH pathology software. S. Chatterjee is a consultant for Biocellvia and has independently verified the results of software analysis described in this manuscript. None of the other authors have any conflicts of interest, financial or otherwise, to disclose.
Figures



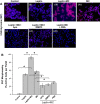
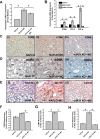
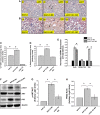
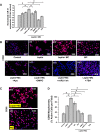


References
-
- Alhasson F, Seth RK, Sarkar S, Kimono DA, Albadrani MS, Dattaroy D, Chandrashekaran V, Scott GI, Raychoudhury S, Nagarkatti M, Nagarkatti P, Diehl AM, Chatterjee S. High circulatory leptin mediated NOX-2-peroxynitrite-miR21 axis activate mesangial cells and promotes renal inflammatory pathology in nonalcoholic fatty liver disease. Redox Biol 17: 1–15, 2018. doi:10.1016/j.redox.2018.04.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

