Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: A randomized, phase 1 study
- PMID: 31393868
- PMCID: PMC6687118
- DOI: 10.1371/journal.pone.0219142
Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: A randomized, phase 1 study
Abstract
Background: Additional forms of pre-exposure prophylaxis are needed to prevent HIV-1 infection. 3BNC117 and 10-1074 are broadly neutralizing anti-HIV-1 antibodies that target non-overlapping epitopes on the HIV-1 envelope. We investigated the safety, tolerability, pharmacokinetics, and immunogenicity of the intravenous administration of the combination of 3BNC117 and 10-1074 in healthy adults.
Methods: This randomized, double-blind, placebo-controlled, single center, phase 1 study enrolled healthy adults aged 18-65 years to receive one infusion of 3BNC117 immediately followed by 10-1074 at 10 mg/kg, three infusions of 3BNC117 followed by 10-1074 at 3 mg/kg or 10 mg/kg every 8 weeks, or placebo infusions. The primary outcomes were safety and pharmacokinetics. This trial is registered with ClinicalTrials.gov, number NCT02824536.
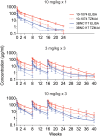
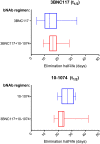
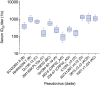
Findings: Twenty-four participants were enrolled in a 3:1 ratio to receive the study products or placebo. The combination of 3BNC117 and 10-1074 was safe and generally well tolerated. There were no serious adverse events considered related to the infusions. The mean elimination half-lives of 3BNC117 and 10-1074 were 16.4 ± 4.6 days and 23.0 ± 5.4 days, respectively, similar to what was observed in previous studies in which each antibody was administered alone. Anti-drug antibody responses were rare and without evidence of related adverse events or impact on elimination kinetics.
Interpretation: Single and repeated doses of the combination of 3BNC117 and 10-1074 were well tolerated in healthy adults. These data support the further development of the combination of 3BNC117 and 10-1074 as a long-acting injectable form of pre-exposure prophylaxis for the prevention of HIV-1 infection.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: There are patents on 3BNC117 (US Provisional Application No. 61/715,642) and 10-1074 (US Provisional Application No. 61/486,960) on which MCN is an inventor. The authors declare no further conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. Epub 2015/09/14. 10.1016/S0140-6736(15)00056-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

